An introduction to Water -- Properties, importance, and the distribution of water

In the past, I had discussed Pressure and its concept as applied to fluids. Most notably air and water. I have also taken my readers on a tour beneath the watery zones of the ocean's depth and as well talked about the various oceans in the world. However, I haven't really described the most important feature of everything I discussed in those posts. - The water itself! I haven't talked about water!- which, I find rather weird.
Hopefully, this post contains all/most of the facts and science behind water that we all need to know.
When we think about water, the first thing that comes to mind isn't the molecular structure or the kind of chemical bonding it undergoes. Rather, it is pretty much definitive by its case use -which is in a lot of areas-. Think about some of its most basic uses - For dehydrating out bodies and quenching thirst, for washing, cooking and bathing to stay clean, for cleaning household items, watering plants, and for many other industrial processes. From a wider view, we really cannot go without water!
Water is super-important to life in general and is recorded to cover about 75% of the earth's surface, down to a depth measurement value of about 3 miles below sea level. But it's not just the planet alone, is it? Even the human body needs water for metabolic activities, and can only last for days without it as the body cells are 2/3 filled with water. With that, it is by far the most abundant substance of the elements and is estimated to be about 3 three times as abundant as all the other substances on the earth combined together!

About 95% of Earth's water is contained in natural land depressions on the surface that serve as reservoirs on the lithosphere. This is known as surface water, and a good example is our rivers and oceans. Water from the ocean is salty, hence, the name 'salt water' or 'saline water'. The remaining 5% is distributed as freshwater (2% in frozen glaciers and ice caps, and as groundwater deep under the soil below cavities and rocks), as soil moisture in swamps and muddy regions, and in the Earth's atmosphere as vapour. More about this in a minute!

So, where did water come from?
Now that we have a rough idea about how much water we have around us, it begs the question; Where exactly did all the water come from? I mean, sure we know that water - like most compounds- is the end product of a chemical reaction between a couple of elements. But we're more concerned about its beginning. How did Earth wind up with so much water than any other place in the known solar system?
As important as that info would be to us, we strangely know so little about the genesis of water. Over the years, we've seen several astronomers and Geo-science 'experts' argue and suggest possible sources of the first water molecules on earth.

While some scientists hypothesized that water might have come from comets and ice-rich asteroids they call carbonaceous chrondrites, Others maintain that since hydrogen was a major element out there in the cosmos, Earth might have retained just enough hydrogen as Deuterium AKA 'Heavy Hydrogen' (an isotope of the Hydrogen atom with an added neutron.) during the expansion of the nebula that formed our star and the rest of the planets in the solar system. And that as the temperature of the earth's lithosphere cooled down, evaporated water in the atmosphere began to collect in depressions on the earth's surface. Some others suggest that water might have been formed deep within the earth's interior via the mechanics of plate tectonics.
So far, we've only been able to explain the science behind water perfectly and as well applied it to our every-day life. The first source of water on earth still remains a mystery to us, but for now, I think we're quite satisfied and grateful for the fact that at least there's enough water to support every life form on earth. We'll simply let the hypotheses and arguments fly around until there's reasonable and solid-enough proof of the real source of water. Perhaps someday, we'll get to the root of it.

Water distribution on the earth.
Moving on from speculations, we do have something to hold on to. As mentioned earlier, Earth's water isn't exactly logged in a particular place in a particular region. Rather, it is unevenly scattered among the four biomes of the earth in varying quantities we measure in percentages. We have water existing simultaneously as a liquid, as gas and as solid in the natural world. This is known as the distribution of water on the earth, and could as well be regarded as the major sources of water even though they are just part of the Hydrologic cycle. They include:
Surface waters.
Surface waters are waters that are visible on the surface of the earth. (i.e; they are exposed to the atmosphere directly). They are mostly saline waters since they are mostly made up of huge bodies of water such as the rivers, lakes, and seas. They provide a habitat for many animal species and as well serve as a source of food and drink. Surface waters are replenished by the condensation of water in the atmosphere.

Groundwater.
Groundwater is simply freshwater below the surface of the earth. As the Hydrologic cycle continues, some amount of water sink into the porous soil to gather in water-saturated zones known as Aquafiers.
Aquafiers are largely made up of rocks, sands, and gravels of different sizes that are permeable enough to allow water flow through them. The exact top level of an aquafier is called the water table, and it has a pressure of 1 atm which means they form the surface of the oceans. Groundwater isn't salty because it is purified as it passes through very fine soil sizes where the impurities are stripped off. Hence, it is freshwater and contains some dissolved minerals. Like surface waters, it is replenished through rain and the melting of snow seeping into the soil. It serves as a good source of drinking water for the greater population, especially in urban and rural areas. It also a good source of water supply for industries that are far away from the sea, but are dependent on the large usage of water. Some good examples also include the Wells, boreholes, and Springs.

Glaciers and ice caps

Freshwater is just 2% of the total water bodies we have on earth, and Glaciers and ice caps make up a greater part of that 2% (with the rest existing as Groundwater).
Glaciers are simply water that is frozen up and are stored as ice mostly around the northern hemisphere of the earth (Primarily in Greenland and Antarctica). They cover about 10% of the earth's Landmass.

The Atmosphere
The atmosphere contains just about 0.22% of the water on earth today. This water evaporates from the surface waters into the atmosphere as moisture through the exposure to the heating effect of sunlight over time, and precipitates to fall back to the earth's surface as dew, fog, and rain!
Every other Source of water -whether saline, fresh or swampy-, falls under these categories!

Properties of water.
Like every other substance on earth, water has characteristic properties which make it distinct from the rest of the other substances. Water has both physical and chemical properties which are largely dependent on how the constituent elements (Oxygen and Hydrogen) combine to form it.
Generally, Elements which do not have an octet or duplet electronic configuration in their outermost shells are always unstable, and would readily go into a reaction where it strives to gain stability through bonding with other suitable elements or with itself (as in carbon). A water molecule, In this case, is produced by the combination of two atoms of Hydrogen, and a single atom of Oxygen. It is a very stable molecule, and this is largely due to the unique bond and structure between the atoms in question.
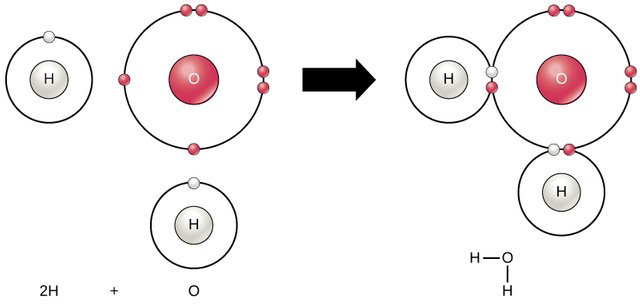
The Hydrogen atom is known to contain just a proton and a single electron in its shell. Hence, it needs a single electron to fill out its orbital and attain the duplet electronic configuration just like Helium. The oxygen atom, on the other hand, is a more electronegative (electron-loving) element because it contains six (6) electrons in its outermost shell, and requires two electrons to fill-out this shell to attain an octet electronic configuration. When these two elements combine, they form a covalent bond, and the valent electrons are shared between them so that the resultant compound attains an octet configuration and becomes stable in its liquid state at room temperature. Thus, Water (H2O) is born.
Quick Water Stats:
Molecular formula: H2O
Molar mass: 18.01528(33) g/mol
Density: 1000 kg/m3 in its liquid state (i.e; > 4 °C). And 917 kg/m3 in its solid state
Melting point: 0 °C = 32 °F = 273.15 K
Boiling point: 100 °C = 212 °F = 373.15 K
Acidity: (pKa): 15.74
Basicity: (pKb): 15.74
Refractive index: (nD) 1.3330
Viscosity: 0.001 Pa s at 20 °C
Crystal structure: Hexagonal -- [Data Source]: Thoughtsco
Other than water being colourless and odourless, below are some specific properties of water.

Polarity of water.
The existence of water as a polar molecule means that there is an uneven distribution of electrical charges and it has partial positive and negative ends. The interaction between these minute charges increases the force of cohesion between its molecules.

When both elements combine, they are joined together by strong hydrogen bonds due to the electrostatic attraction between the atoms. The 2 hydrogen atoms are attached to the oxygen atom at exactly 104.5o apart from each other, giving it two positive charges. While the opposite end of the oxygen atom has 2 negative charges.

Adhesion and Cohesion.
The polarity of the water molecule also gave rise to the property of cohesion and adhesion. The cohesive of water molecules means they're attracted to other molecules of water as well. The water molecules form hydrogen bonds readily with the neighbouring water molecules, making it remain as a liquid at room temperature. A good example of this effect is property is Surface tension!
Adhesion is very similar to cohesion, but with a little difference because it involves the attraction of a molecule to other kinds of molecules. Water is adhesive to other molecules with one condition: The molecule should be able to form hydrogen bonds with water. A good example of the effect of adhesion is capillary action (which is the ability of a liquid to rise in a thin capillary tube). The water is seen to stick to the glass around the edges, and then slope down to a low area. We call this the upper and lower meniscus respectively.

The universal solvent.
An excellent nickname for water is ''the universal solvent'' which is really apt, considering the fact that it can dissolve far greater range and quantities of matter than any other solvent yet known, and it is a life-giving constituent in many other substances and even solvents as well (whether polar or ionic solvents).

Water's unique 'solvent power' is as a result of its polar nature. The charges at both ends allow water to be easily attracted to other compounds, to break them up to their constituent elements and then dissolve them over time.source Take Sodium Chloride NaCl for example - our table salt, and a good source of iodine. Sodium ions are positively charged while the Chlorine ions are negatively charged. When NaCl is put in water, the Oxygen in water splits and goes into reaction with the positively charged Sodium ions, while the positively-charged Hydrogen ions combine with the negatively-charged Chlorine ions. The result is that with time, the constituent elements of the NaCl compound are pulled apart and the salt is seen to dissolve in the water to form a transparent homogeneous solution.
But of course, there's a limit to water's solubility, which is measured as its saturation. The solubility value of water isn't a static one, but is rather dependent on a number of factors like the temperature of the water and the pressure.
Taking our salt for example again, as more NaCl gets added, the water dissolves as much as it can until it isn't able to dissolve any more salt ions. At that point, water is said to be saturated as there are no more free ions to dissolve the newly-added salt ions. So they remain in the solution as solids. This is really important because it proves that water doesn't dissolve just about anything simply because it is wet. Rather, water dissolves compounds because of the reaction and science behind it. In the end, the salt isn't 'lost' in dissolution, but can be retrieved by several methods such as heating the solution to dryness to reveal its crystals.

Free form, Incompressibility, and transparency.

Just like any other liquid, water has a definite volume and is not readily compressible like gases are. This is because the molecules in liquids are a bit closer to each other, and they have a stronger intermolecular force of attraction between them in comparison to gases which are homogeneously scattered. and move even more randomly. Hence, water has a definite volume and would always take the shape of the container it is put into.
Also, water is transparent to light and other forms of electromagnetic radiations. This makes it easy for the human eye to see through a glass of water, and see things below surface waters clearly to a certain depth. A significant amount of sunlight can travel through ocean water up to a depth of about 650 feet.

Phase change in water.
Water is the only substance in our planet that naturally exists in all three states at once. A good 3-in-one example would be a lake in the icy regions or during winter, where there's ice (water in its solid state) on the surface of a lake, liquid water below the ice thickness, and gas (or vapour) in the atmosphere above the ice as sunlight reaches the lake's surface constantly.
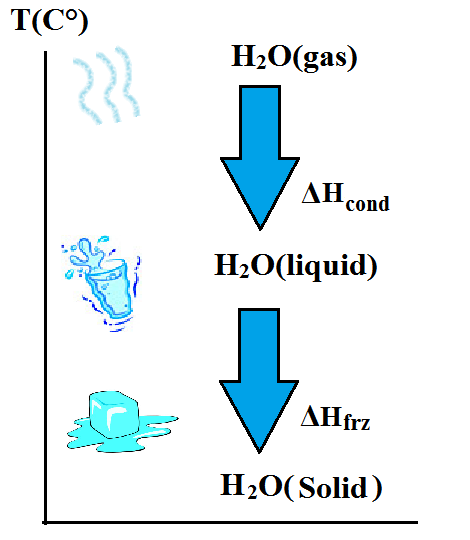
Through the application and removal of heat, water undergoes what we know as phase changes. It turns into ice at 0oC, remains in its liquid state above that temperature, and turns into vapour at 100oC. These points at which water turns into ice and vice versa, and into vapour and vice versa, are known as the melting/freezing point, and the boiling point respectively.
Phase changes in fluids occur without any net change in temperature of the fluid in question and normally requires a minimum energy condition that must be met before it happens. Since water has very high specific heat capacity, it would require a large amount of energy to effect these phase changes. Take for example the phase change from ice to water. The energy involved at this phase is termed the latent heat of fusion, and it is the amount of heat energy required to melt 1 gram of ice into 1 gram of water while the temperature still remains constant at 0oC. For pure water, this energy required for the phase change from solid to liquid is exactly 80 calories (not to be confused with Calories with a big letter 'c') and is released as the Latent heat of crystallization whenever water freezes back into ice.
This value isn't the same for the Latent heat of vapourization, which is the amount of energy required to change 1 gram of water at 100oC into 1 gram of water vapour at the same temperature. About 540 calories of energy are required for this process, which is almost 7 times greater than the latent heat of fusion. It should also be noted that the same 540 calories are liberated when vapor condenses to liquid again. The heat involved this time would be called the latent heat of condensation.

Anomalous expansion of water.

Another unique characteristic that sets water apart from other substances is its behaviour as it approaches its freezing point of 0oC. At 4oC, water reaches its greatest density and starts expanding upon freezing, thus, reducing in density. This is quite unusual since the effect of a decline in temperature for most substances leads to the compactness of the molecules and a corresponding increase in density. However, that is not the case for water, and this simply indicates that ice would float on water since ice is less dense. This phenomenon is responsible for the freezing of lakes from the top rather than from the bottom where it's colder.

Specific heat.

The specific heat of water (i.e, the amount of heat energy required to raise a gram of water by 1oC) is very high for water relative to other substances. What that simply means is that water is a poor conductor of heat, and much more heat energy (about 10x the amount) would be needed to raise the temperature of 1 gram of water, than it would take if you were to heat up harder substances such as Iron (Fe), copper (Cu), dry soil or Zinc (Zn). For this reason, areas closer to large bodies of water such as the ocean, have relatively lower temperatures when compared to areas that are far away from them, regardless of the fact that they receive the same amount of sunlight. This is because the water body around those areas moderate the temperature changes in the air as they absorb most of the heat to evaporate and are carried about by the prevailing winds from sea to land.
Also, such areas hardly witness any reasonable temperature changes at all during marked seasons like summer and winter. The water molecules of the ocean absorb large amounts of the heat energy from the sun and still doesn't considerably warmer.

Mobility of water.
Water molecules are also extremely mobile in its liquid and gaseous phases of matter, and this makes it a good medium for the transportation of large quantities of heat energy from one point to another. For example, the action of deep-water currents (upwelling and downwelling) in oceans could move or transport individual molecules of water to different places around the world. The glass of water on your desk may contain a molecule of water that was formed over 100 years ago. However, it might have gone through countless water cycles before being collected and purified for drinking.
Also, as far as mobility is concerned, water could take up energy and evaporate at a lower latitude and then give up that energy at a higher latitude and condense. Ice may form at an altitude and then gain heat energy and melt at another altitude. It is an endless cycle, and in the end, no water molecule is truly lost.

Surface tension.
Surface tension is one property of water that has found ready applications both in nature and in the general daily activities in the human society. We've witnessed surface tension as little insects 'walk' on water without sinking, and we've applied the principle to detergents for washing, clinical urine tests and rainproof materials as well. The list seems endless.
By definition, Surface tension is the ability of the surface of a liquid to behave like a stretched-out elastic. This is simply due to the force of cohesion between the liquid molecules, which, for water, is the direct consequence of its polar nature. The resultant effect of surface tension is that the surface of water forms a film and becomes a bit more resistant to penetration by an external body. Surface tension decreases as temperature increases and the molecules of water gain energy to move even more randomly. It is measured in dynes/cm. The dyne is simply the force it would take to break through a film of water 1cm long. At 25oC, the surface tension of water would be about 72.86 dynes/cm in comparison to 22.3 for ethyl alcohol and 465 for mercury at the same temperature.source

Final words, and the importance of water.
In conclusion, Earth isn't known as 'the water planet' for nothing, and its existence as the only planet with the presence of life forms yet known owes it all to water -the matrix of life-. The importance of water is overwhelmingly important because every living thing cycles water through their bodies and need it for growth. Humans are about 70% composed of water, which carries out important functions such as regulating body temperature, hydrating the brain and lubricating body joints, aiding body metabolism and ridding the body of toxic wastes.
As mentioned earlier, water moves in an endless cycle in our planet as evidenced by the water cycle. The water cycle ensures that we are not sintered under the sun. It keeps the atmosphere moist, it regulates weather patterns, and keeps our climate moderately stable. Dry seasons are notable for the absence of natural precipitation such as rain while rain seasons see plenty of showers. However, our bodies are not left out too, because we take in water by eating and drinking, and then release them back into nature by perspiration, by exhaling as vapour and through our urines as well.
Water also affects other areas of life as well. Agriculture depends on water for the needed nutrients for the growth of plants, Water provides a natural habitat for aquatic life to thrive in, Rivers and seas serve as a medium of transportation for heavy goods when we use ships and ferries, thus boosting tourism and economics.
Water is life. What more can we ask for?
Thank you for reading
References
[Livescience -- Where did water come from]
[NWGA -- Earth's water]
[Wikipedia -- The water cycle]
[Wikipedia -- Water distribution on the earth]
[Smithsonian -- How did water come to earth]
[Thoughtsco -- Why water is a universal solvent]
[SparksnoteAtomic structure of H2O]
[Aquafil -- Natural sources of water]


Good job, a detailed article and a lot of information and your explanation of polarization was very good and simple. i have just a simple addition:
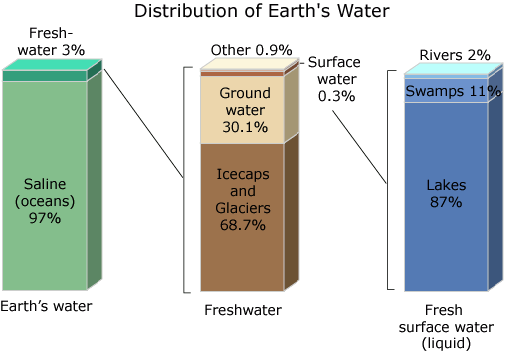
You said in your article that there is enough water to support life on Earth, Yes, you are right, but I disagree with you about how satisfied you are about this because freshwater who is drinkable directly Without processing represents only 3 percent of the total amount of water on Earth
97 percent of the earth's water is in the oceans needs chemical methods to make this water drinkable, as these are often expensive and some countries can not afford, this is why drought is a concern for many governments and peoples.
A World Bank report on development says water shortages threaten 80 countries, 40 percent of the world's population, more than 2 billion people are suffering from basic human needs and the most basic public health rules and governments around the world have recognized the potential of water infrastructure to complement other economic and social policies, including those aimed at improving health outcomes. It is no coincidence that one of the world's largest lending portfolios includes investments of $ 35 billion in water projects.
:)
Excellent feedback from a rearry attentive reader. Sad isn't it? that with all of the water available, such a large amount of it isn't drinkable and requires purification methods.
Which is why we can ever be grateful for the hydrologic cycle, nature's way of purifying our water. Enough, No? but we hope we get water t those places where there's less of it within the coming years.
Voted for visibility.
The water is common everywhere, but it has some interesting properties. Some of us take it for granted when we open a faucet and the water runs out, clean and safe, for our consumption. Somewhere in our continent, a child will have to trek 20KM to get 10 litres of water; which is twice what we waste when we leave the showers running for about two minutes.
Awesome contribution. Cheers
wow. that's some interesting fact. I once listened to a TEDx talk by Marla Smith Nilson about how a group of settlers in Africa would trek 4 hours to get water each day. Sad thing was, the water source was just a pond, shared between animals directly. Convenient? No, Clean, No! Suffcient to take home? No.
For those who waste it, well, i suppose Surplus breeds abuse
Congratulations! Your post has been selected as a daily Steemit truffle! It is listed on rank 2 of all contributions awarded today. You can find the TOP DAILY TRUFFLE PICKS HERE.
I upvoted your contribution because to my mind your post is at least 16 SBD worth and should receive 220 votes. It's now up to the lovely Steemit community to make this come true.
I am
TrufflePig, an Artificial Intelligence Bot that helps minnows and content curators using Machine Learning. If you are curious how I select content, you can find an explanation here!Have a nice day and sincerely yours,

TrufflePigWe just drink water and don't care about how it came about and sometimes I feel it's nature despite the science and chemistry backing it
Thanks for the eye opener
You're welcome buddy. And hey, if ever you formulate your own hypothesis on the beginning of water, I'd love to hear all about it.
I have learnt from this man
Lol how would I
Water i life really, to find any sort of life outside our planet we need to find water there first and that is why NASA in their search for extra-terrestial life is doing, water first then life follows.
Nice post. A good summary of water properties.
Thanks buddy.
But this post is long o...
Surprisingly long.
Wow what a good collection of information about that which keeps us all alive. Fascinating to know that 20% of the global fresh water is in the great lakes right near home here.
Also there is no beer without water so that is of tantamount importance!
Thanks for compiling and presenting so elegantly.
Zeke!!! 🤣😎
Beer = Life!
This post has been voted on by the steemstem curation team and voting trail.
There is more to SteemSTEM than just writing posts, check here for some more tips on being a community member. You can also join our discord here to get to know the rest of the community!
Hi @pangoli!
Your post was upvoted by utopian.io in cooperation with steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV