THERMODYNAMICS BASED ON ITS LAWS
Thermodynamics is a branch of physics that deals with temperature and heat in relation to energy and work. This really entails about energy conversion and direction of change i.e the study of energy change.
There are four laws governing thermodynamics which are
(1) Zeroth Law of Thermodynamics
(2) First Law of Thermodynamics
(3) Second Law of Thermodynamics
(4) Third Law of Thermodynamics
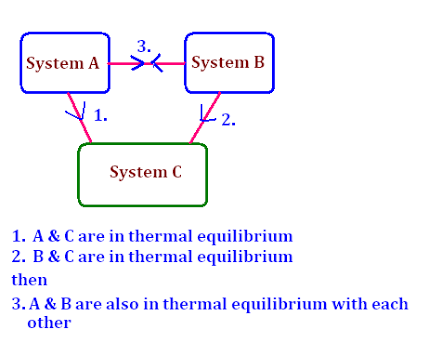
ZEROTH LAW OF THERMODYNAMICS
This law states that if two bodies are in thermal equilibrium with a third body, they are also in thermal equilibrium with each other.
That is we can say if body A and body B are in thermal equilibrium with body C....A,B&C are in thermal equilibrium with each other. This law deals with thermal equilibrium and provide a means of measuring temperature
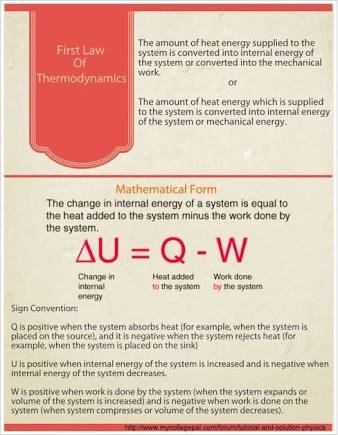
FIRST LAW OF THERMODYNAMICS
This law states that the change in the internal energy ΔU of a closed system is equal to the amount of heat Q supplied to the system, minus the amount of work i.e workdone by the system on its surroundings.
This law has its limitation that is we can deduce some facts.
- A closed system that undergoes thermodynamics cycle, its net heat transfer is equal to the workdone. This does not specify the direction of flow of heat and work
- Mechanical work and heat energy are not mutually convertible which implies that only mechanical work can be fully converted into heat energy while only a part of heat energy can be converted to mechanical work.
SECOND LAW OF THERMODYNAMICS
There are only two ways to define this law which are Kelvin Planck Statement and Clausius Statement.
KELVIN PLANCK STATEMENT
This states that it is impossible to construct an engine working on a cyclic process whose main is to convert heat energy from a single thermal reservoir into equivalent amount of work.
This law is also known as Law of Degradation of Energy
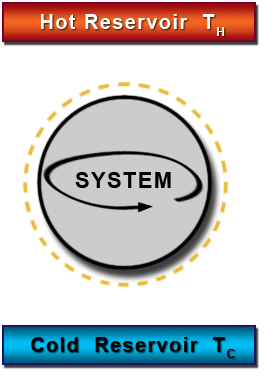
HEAT ENGINE
This is a device used for converting heat into mechanical work, there should be at least two reservoir of heat, one at higher temperature and the other at lower temperature.
THERMAL RESERVOIR
This is a body of infinite heat capacity which is capable of absorbing or rejecting and unlimited amount of heat without affecting its temperature such as atmosphere and ocean of water.
CLAUSIS STATEMENT
This states that it is impossible for a self acting machine working in a cyclic process to transfer heat from a body at a lower temperature to a body at a higher temperature without the aid of an external agent for example Refrigerator or heat pump.
REFRIGERATOR
It is a device which operates in a cyclic maintains the temperature of a cold body(refrigerator space) at a temperature lower than the temperature of the surrounding
HEAT PUMP
It is a device which operates in a cyclic process maintains the temperature of a hot body(heated space)at a temperature higher than the temperature of the surrounding.
• The performance of refrigerator and heat pump is measured in terms of coefficient of performance which is defined as the ratio of the maximum heat transfer (that is heat taken from the cold body) to the amount of work required to produce the desired effect.
THIRD LAW OF THERMODYNAMICS
This law states that the entropy of all the perfect crystalline solids is zeros at absolute zero temperature.
The third law of thermodynamics is also referred to as Nernst law. It provides the basis for the calculation of absolute entropies of the substances.
Post is original except pictures that have been properly sourced and laws which have been quoted.
.jpg)
.jpg)
.jpg)
.jpg)
.png)
.jpg)
.jpg)
.png)
.jpg)
I will suggest you join the steemstem discord server.
Awwwwn....tnks dear
I will suggest you join the steemstem discord server.