SEPARATION PROCESSES IN SCIENCE AND ENGINEERING
A Separation Process is one in which matter is transferred from one phase to another across an interface under the influence of a natural driving of a natural driving force such as diffusion.
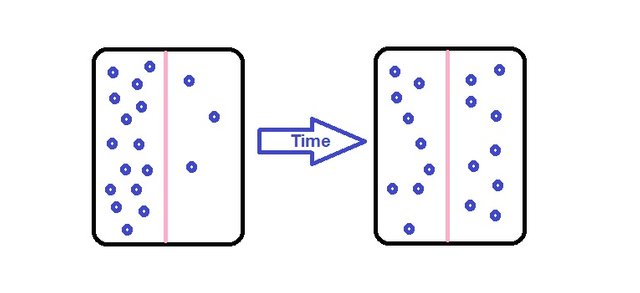
Diffusion is a famous term we might all be familiar with. Diffusion can be considered a phenomenon which molecules are transferred from a region of high concentration to another region of lower concentration to achieve an equilibrium state. So with that definition we can see how it can tend to be a force to aid a separation process.
Another key term involved in separation processes is Mass transfer.
Mass transfer is the tendency of a component in a mixture to travel from a region of high concentration to a region of low concentration, basically dealing with composition of masses.
Mass transfer operations are concerned with changes with compositions of mixtures and are used in separating and controlling the processes phase are involved.
EXAMPLES OF SEPARATION PROCESSES
Separation processes are numerous ranging from basic Drying down to Fractional distillation (a very famous practice in Refineries). Examples of separation processes include;
Drying
Absorption
Adsorption
Leaching
Ion Exchange
Distillation
Gravity
Filtration
Evaporation
Stripping
Humidication/ Dehumidification
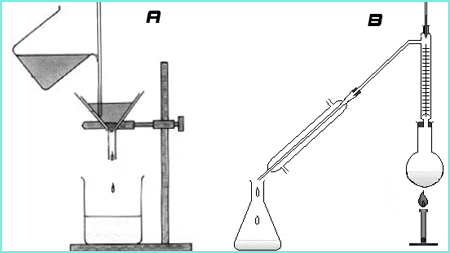
A is Filtration while B is Distillation
Image Credit
DRYING
Drying may be defined as the vaporization and removal of water or other liquids from a solution suspension, or other solid liquid mixture tonforn a dry solid. This is kind of a complex process, more technical than the basic drying (surface information we alll know). It involves simultaneous heat and mass transfer, accompanied by physiochemical transformers.
For example, when we dry our washed wet clothes out in the Sun, the Sun’s heat draws out the liquid(water) to the air(heat transfer) and then the air carries the water molecules into the armosphere(mass transfer). Hence the physiochemical process.
ABSORPTION AND ADSORPTION
Absorption is a physical or chemical process in which atoms, molecules or ions enter some bulk phase – liquid or solid material. It is different from adsorption as involves gases and liquids wholly while the latter involves solids.
They both deal with taking in gases or liquids.
As for Adsorption, it basically involve solids. It is the adhesion of atoms and molecules from gas and liquid streams into solid surfaces. Absorption is the process in which something takes in another substance into itself.
LEACHING
Leaching is the process of extracting substances from a solid by dissolving them in a liquid, either in nature or through an industrial process. It is mainly concerned on the porosity of the materials involved. Any material exposed to water can be leached.
FILTRATION
This is a well-known separation method and process. It is a process in which solid particles in a liquid or gaseous fluid are removed by the use of a filter medium that permits the fluid to pass through but retains the solid particles.
DISTILLATION
Distillation is a process of separation the components in substances from a liquid mixture by selective evaporation and condensation. In more understandable terms, it is a separation process that involves separation of miscible materials by addition of heat and manipulation of temperatures. It may result in essentially complete separation or partial separation. In either cases, the process involves differences in the volatility of the mixture component. So we can draw that distillation is concerned with separation of solutions where all the components are appreciably volatile.
Note that distillation can be carried out by either two methods which are;
- In the first method, the vapor produced by boiling. The liquid mixture to be separated is condensed without allowing any pet of it to return back to the still(the distillation apparatus), there we can say that there was no reflux involved.
- In the second method, a portion of the condensed vapor is returned back to her still where it comes in contact with the vapor on its way to the condenser.
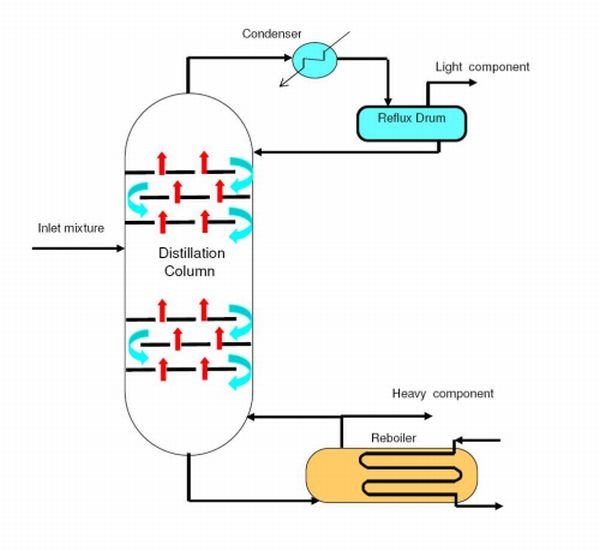
A Distillation Column
Image Credit
CONCLUSION
Separation processes are very important to Chemical industries and also a very vital part of the science and engineering professions. In Chemical Engineering,a separation process must be involved. We can even go as far as the pharmaceutical industries, separation processes are crucial too. In summary, anywhere we need to purify substances, a separation process must be sourced out and utilised.
REFERENCES
- https://www.britannica.com/science/filtration-chemistry
- https://en.m.wikipedia.org/wiki/Adsorption
- Unit Operations for Chemical Engineers, Warren L. McCabe, fifth edition, 1993.

i ve learnt alot from this article on seperation techniques. thanks alot.
this should help me a long way in one of my chemical engineering courses
Very informative bro. Such a wonderful reminder
@originalworks