Radioactivity: Definition, measurement, life expectancy, source, use, reaction, remedies and treatment
Radioactivity
Although the term is a science, in 1986, Chernobyl attracted the attention of common people for the first time after the accident. Recently, "radioactivity" has become the main issue of discussion after a nuclear reactor accident in Fukushima. But sadly, most discussions (magazines, blogs and forums) radioactivity are sometimes exaggerated, sometimes vague, and sometimes misinterpreted. To understand the radioactivity, there is no need to be a student of science, the basic idea about this is the desire and readiness of knowing one's consciousness.
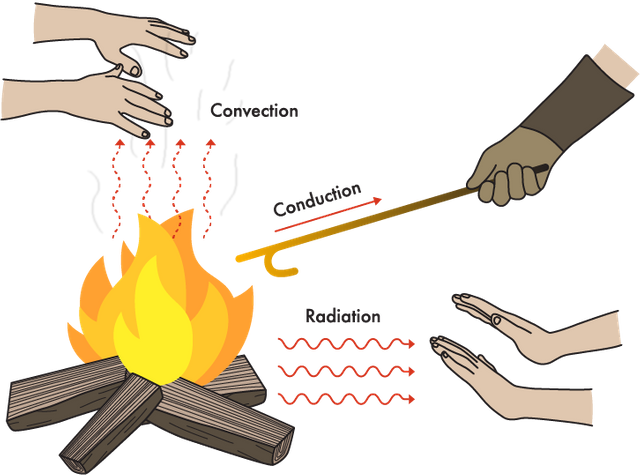
What is radioactivity?
In 1896, French scientist Henry Bakerrell invented the first known invention of a special particle of energy that emitted from the nucleus of the uranium, continuously spontaneously. Later it was found that the inorganic isotopes spontaneously transmitted different types of rasani and converted into their nuclei and turned into isotopes of other substances. This behavior or religion is called radioactivity. Such isotopes are called radioactive isotopes. Uranium-236, Stronsium-90, Iodine-130, Plotonium-236 Ultracable radioactive isotopes. Nuclear phenomena of radioactivity atoms, so it can not be controlled by any outside physical process.
We know that every substance is made up of very small particles called atoms. Atoms of all elements have electrons, protons, and neutrons - these three main particles. The center of the neutron and proton atoms lies in the nucleus. The electron is out of the nucleus. Nuclear is not fully charged. Neutron chargeless, the number of negative electrons and positive protons on the atom is equal. There may be several types of mass atoms of the same element (mass = total number of protons and neutrons). The atoms of atoms that are equal, but the mass numbers are different, the atoms are called each isotopes. That is why the creation of isotope is different for the number of neutron numbers.
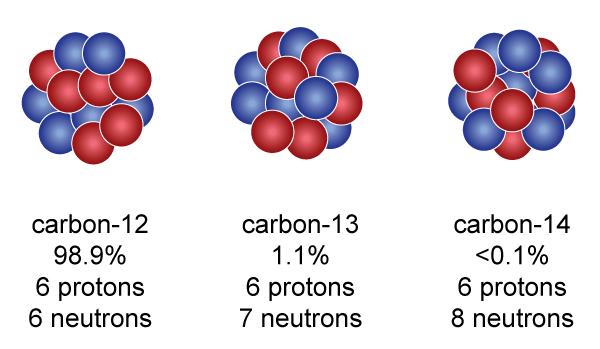
In the figure above the three atoms of carbon - the number of protons are the same, the difference is just neutron, 6, 7 and 8 respectively. So these isotopes of carbon. Most of the natural environment of the planet is carbon 12 Physical and chemical properties of all isotopes of the same element are the same, but there is considerable difference in the behavior of the behaviors. Such as carbon-14 stationary and radioactive. Isotopes can be natural or artificially produced.
To make it easier, radioactivity is the spontaneous release of energy from unstable atoms. These strengths are both type of massed particle ray or amplified electromagnetic ray. Radioactive radiation rays are mainly of three types: alpha beta and gamma rays.
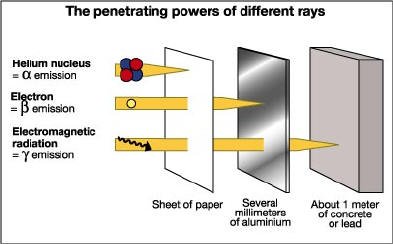
Alpha (a)
particles consist of two neutron and two protons. It is comparatively heavily charged with heavy and positive radiation. According to the structure it can be called helium nucleus. Alpha radiation can not exceed 2 cm in air. Even the slightest paper barrier can not cross the alpha. Therefore, it is not harmful for people in the direction of distance. However, if an object is transmitted in alpha, it may be possible to cause serious damage (which is about 3 to 20 times more likely than other rays) to enter the human body directly or indirectly.Beta (ß)
particles are very light, which do not have much weight. They are like electrons in characteristic traits. These are actually intense speed electrons. The beta particles are negative and the power to cut them is 1000 times more than the alpha particle. They can go up to a few feet in the air and people can penetrate the skin's outer strings. Diseases of the skin can be caused by a large amount of beta-cholera, even skin cancer.Gamma (?)
is not a particle, it is a light-like electro-magnetic wave. So their speed is about 300,000 kilometers (186,000 miles) in seconds Their cut capacity is very high. They can navigate through various materials like dyeing rays. It is possible to keep gamma Rasni with only a single lead or concrete wall wide wall. They damage the living cells. This gamma ray is mainly responsible for radioactive eruptions due to the adverse effects of exposure to biodiversity and vegetation in the vast areas. Moreover, after taking any gamma ray, some particles of unusual radiation are harmful for human health.The waves of cosmic, gamma, dye, ultraviolet, visible and infrared rays and micro, radio, and television waves, such as the electricity-magnetic radiation wave. Not all are harmful to humans. Their main differences are the wave-length and the vibratory numbers. The visible light is just a small part of the power-magnetic radiation ray. Most of the radiation energy is invisible.
Measurement of radioactivity
No amount of radioactive radiation from the nucleus of billion billion atoms of any substance is not in a precise rhythm, simultaneously or at the same speed. Generally, the amount of radiation is determined by the number of radiations (or per second or second) of radiation, or by the level of energy emission. Various units and dimensions are used in measuring radioactivity measurements. Because from the beginning of radiation to the end, three steps: the source, the radiation beam and the absorbing.
The radiation and radiation measurement is done in three ways for three-phase (Radioactivity, Absorbed Dose, Effective Dose). When the matter of radiation comes in relation to nuclear reactor and radioactive elements, the amount of radiation from the source is seen. This amount is expressed with bakrel or curie. A radiation in every second is called becquerel. 37,000,000,000 radiation per second is called 1 curie (1 g of radium-266 leaving 1 quarry radiation in one second). Based on the distance from the source, the age of man, the physical structure, the radiation grade, time, etc. radioactive response can be different to the person and the object. The amount of radiation absorbed in different substances (Absorbed Dose) is used for the use of red (rad) or gray (gray). When it comes to health, medical and environmental issues, then how effective is the quantity of the effective dose after the absorption of absorbing doses after the absorption of radiation. The amount of this dosage is expressed with rem (se) or sievert. If the absorbed radiation in the human body is beta, gamma or x-rasna, then 1 gray = 1 dose = 1 sievert is caught. And if alpha is Rasna then 1 gray = 1 dose = 20 sievert is caught. It means that if a human body accepts a dos and a dosage of alpha-ray of beta / gamara one gray, it will be 21 sevart.
If we consider rain as radiation to better understand the radiation measurement, and if we wet it in the rain, then the rain volume (Bq), the amount of rain that fell on our bodies, the amount of gray (gy) and the part of our body in the rain. Sv) can say.
Rem, Red and Curie old units that are no longer used. The International System of Units (or SI units): gray, sievert, and becquerel.
Here's a list of comparative concepts:
1 curie = 3.7 x 1010 disintegrations per second
1 becquerel = 1 disintegration per second
1 millicurie (mCi) = 37 megabecquerels (MBq)
1 rad = 0.01 gray (Gy) = 10 mGy
1 rem = 0.01 sievert (Sv) = 10 mSv = 10000 µSv
1 gray (Gy) = 100 rad
1 sievert (Sv) = 100 rem
1 coulomb/kilogram (C/kg) = 3,880 roentgens
1 roentgen (R) = 0.000258 coulomb/kilogram (C/kg)
The duration of radioactivity
Age of radioactivity
Although the science of 'half-life' or 'half-life' is known to science students, it is often misconstrued to ordinary people. To know how much radiation can be from a radioactive element, 'Half-life' or 'half-life' in the language of science must be understood. We know that radioactive substances can be dissolved in different types of rasni and they are transformed into nuclei to become non-oxygenic substances. Meaning radioactive material is consumed due to radiation with time. For the amount of radioactive material consumed by any amount, radioactive erosion, half the amount of time to be half-life is called. For example, see the graph of carbon-14 radioactive isotope:
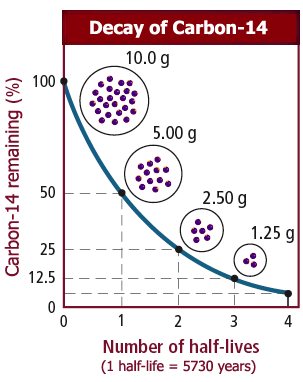
If there is 1 gm carbon-14 at the beginning, it will take 5730 years to become radioactive-consumed and become 0.5 grams. It will take 5730 years to take the 0.5 grams in the same way again to become 0.25 grams. Because of this, half-life of carbon-14 = 5730 years. Thus, it will take about 5000,000 years to become fully-consumed (actually turning into C-14 to N-14) 1 gram sensitive carbon-14. Many people may think half-life = 5730 years when full-life = 11460 years! Wrong! According to the scientists, the concept of time required to react all of the radioactive material is meaningless, because the reaction moves very slowly and when only a small quantity of the object remains, then it will be necessary to indefinitely end the reaction.
Half-life listing of some isotope known:
Nitrogen -16: 7 seconds
Iodine -131: 8 days
Phosphorus-2: 14 days
Cesium -137: 30 years
Plutonium-233: 240000 years
Uranium-2335: 704 million years
Potassium-40: 1.3 billion years
Rubidium-87: 49 billion years
(Source: http://www.iem-inc.com/toolhalf.html)
The source of radioactivity
The source of radioactivity can be divided into three categories:
- Natural,
- The creation of humans and
- Environmental surroundings
Natural Source:
All the Earth's electron, proton, and atomic nucleus at some of the atoms of the Earth have been struck by light at almost every moment. These are called cosmic rays. These rays are electrically charged and transmitted in a small amount of atmosphere to the surface of the earth. The higher the height of the surface of the surface than the sea level, the greater the level of its growth. Earth's radiation means that the electricity and magnetic radiation emanating from the Earth and its atmosphere is also a source of radioactivity. There are still some amount of radioactive elements that existed when the world was created. Because their lifetime is very long. Such as K-40, U-238 and Th-232 etc. Radon Gas is the most important source of natural resources. It is 8 times heavier than the wind. It has three natural isotopes. These are caused by radioactivity of radium, uranium and thorium. Apart from natural rock, plants, animals and various industries, radian gas is added to the atmosphere. Generally, a person is experiencing three millimeter secrete natural radioactivity annually.Man created:
The level of radioactivity created by humans is varied according to space and time. X-rays and other radiation therapy machines are a major source of radioactivity in treatment. Various components are used to increase the production and quality of industries in the industrial sector, from which dangerous holes are created. The radiation emitted from the nuclear reactor and the atomic massacres in the public areas are dangerous for people in enormous areas. Besides, a large number of radioactive substances from the Nuclear Power Plant (Chernobyl and Fukushima) have spread to large areas around the area.
3. Environmental environment:
Almost every thing around us is getting radiation. Our homes, food, drinks, even radiating radioactive rays from our own body.
This radiation is very low, which is not harmful to humans. Depending on the environment, its rate may be higher. As the brick-built house radiation levels are more than the wooden house.
Use of radioactivity
For the last decade, people have made significant improvements in the nuclear branch of science. Many artificial isotopes have been created for different needs. Nuclear power is used in making nuclear weapons, generating electricity, in complex treatment, and in industrial factories. Electricity is produced by turbine and generator using the heat energy generated by the fission process from the radioactive isotopes at the nuclear power plant.
Cancer treatment was done by the death of cancer cell in gastric ray of radio therapies. Radiation plays an important role in the process of X-rays, mammography, angiography. Radioactive objects are used to conserve medicine and medical equipment. The use of radioactive materials for the study of various acute diseases including AIDS, cancer.
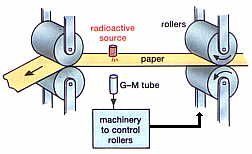
Alfa and beta rays are used to calculate the exact amount of thickness, concentration, and element of various objects such as paper, plastic, aluminum etc. in construction or production industries. If there is any crack in the liquid or gaseous substance driven by the pipeline using the very small amount of radioactive material and the geostationary pipe line, then it can be determined from the surface of the soil. To make it visible in the dark, the zigzag sulfide mixed with the radioactive Thorium in the clockwise and the number is plated.
Radioactive rays are used to improve food quality, conserve, increase nutrients and reduce pest. Due to genetic mutation, radioactive radiation is applied to produce more yielding, adverse weather conditions and insect pests. It is possible to determine the amount of soil, water or air pollution by using a small amount of radioactive material that is not harmful to humans.
By analyzing the carbon dating method (radioactivity-measurement measurement), it is possible to analyze fossil samples and age of rocks, analysis of plants' internal chemical and biological process analysis, artificiality and other artifacts and the physical and chemical properties of minerals. Radioactive material used in scanning machines and smoking detectors for safety.

Radioactive response to living organisms
A small amount of radioactivity does not harm us. Because our body has natural resistance. In addition, if there is any cell in our body, it will automatically recover in many cases, if the cell dies, then new cells are formed in the biological process. How much radiation is harmful to humans can not be precisely defined. Because it can be different for each other. The effects and levels of radioactivity on humans depend on three conditions: the time of radioactivity in the area, distance from radioactive sources and preventive measures. No safe-range has been discovered or recognized yet, because the tolerances of different people vary. However, it is generally considered that no one has ever received more than 1 (one) mile-sever in radiation.
Normally, the amount of radioactivity emitted in X-ray is 20 microforteways. Generally, the level of radioactivity used in the therapeutics of different therapies in diagnosis of disease is far more than natural radioactivity. Once the X-ray receives a person's exposure to radioactivity, it is equivalent to about 10 days of natural radioactivity, whose effective levels are about 0.1 ml of sewart. The amount of radioactivity used in IVP or intravenous palmogram test for kidney and bladder testing is equivalent to one year of natural radioactivity.
Effects of radioactivity in humans
Not all tactical substances react to the same. If excessive radioactive substances enter the body or come in touch then we may face different types of physical and mental problems. When radioactive rays come in contact with people, the living cells of the body are destroyed or there is abnormal behavior in the cells. Alpha and beta rays react to the outer part of the body and the formation of gamma ray cells. Although the amount of radiation is slightly, it may be harmful to humans due to longer periods of time. Again the amount of radiation is slightly higher, but due to a short period of time it may not be harmful to humans. The risk of radioactivity is more likely to cause cancer and leukemia. Moreover radioactiveness is also responsible for the physical and mental dysfunction of newborns and children. There are numerous reactions in our human body due to the adoption of radioactive radiation in one day. Though it differs on physical ability, however, the following two lists may be considered for the general idea of ??general ability.
As a single unit of sewart, the response to radioactivity levels can be taken in one day:
- Levels 0 - 0.25 SV (0 - 250 mSv): Fully secure, nothing will happen.
- Dimensions of 0.25 - 1 Sv (250 - 1000 mSv): Those who are physically weak, some of them will have unhealthy, nausea, hunger and depression. Pain or abdomen or abnormalities can be seen in someone's bone-spots or lymph nodes or in different parts of the body.
- Dimensions 1 - 3 Sv (1000 - 3000 mSv): Uprooted food will cause nausea, nausea, hunger, pancreatic injury, bone marrow or lymphatic pain, or pain in different parts of the body. Dense, depression and abnormalities will be felt. If treated properly, they can be cured of almost all cases.
- Level 3 - 6 SV (3000 - 6000 mSv): There will be plenty of vomiting and hunger-depression. Blood stains, wounds, diarrhea, various types of skin diseases and skin scars will appear. If not treated immediately, death is inevitable.
- Level 6 - 10 SV (6000 - 10000 mSv): Negro tumors will collapse with the above symptoms. The probability of death occurs within a short time
- Dimensions exceed 10 SV (10000 mSv): Physical numbness and inevitably death.
Radioactivity and treatment
As mentioned earlier, the radiation may be slightly harmful, but it may be harmful for people due to longer periods of time. Again the amount of radiation is slightly higher, but due to a short period of time it may not be harmful to humans. It is almost impossible to avoid natural and environmental radiation. It is almost possible to avoid some of the man-made radiation when it is little aware. If you reduce the harmful levels of radioactivity on yourself, you must emphasize on three subjects: time, distance and reserve cover.
Minimize Exposure Time to Minimize Dose
Maximize Distance to Minimize Dose
Maximize Shielding to Minimize Dose
We need to protect ourselves from radiation and harmful radiation:
- Staying out of keen sunscreen (12-2pm noon).
- Try to avoid rainwater as much as possible.
- Consume food rich in chlorophyll and oxidant.
- Use iodine in food (iodized salt).
- Use sun-glass when you go out of the day.
- Do not take too many radiation therapy over.
- Stay away from all the machines that are X-ray and radiation.
- When you go out outside the day, use body cover or sun-lotion or cream.
- Awareness about the side effects of the chemical substances used in household work in daily life.
- Use the radiation detection device at laboratory and industrial factory workplace.
- Try to consume less fruits that are mixed and marketed by mixing chemicals directly.
Although the lowest harmful levels of radioactivity can not be ascertained, yet the safety rate of human radioactivity is considered to be 1 (one) milli-sewer.
Final word
Cancer is one of the most common disorders we face due to global climate change, environmental pollution, unhealthy environment, chemical use and adulterated food. So there is no way to deny the radioactivity that we are aware and unknowingly. Cancer is a disorderly disorder. Moreover, its treatment is very complex and costly. Although radioactivity can not be completely avoided, but if we take a few warnings, we can reduce its levels very much. Awareness and remedies needing treatment before all radiation seeps with cancer.
You can learn more from thes site given below:
- Wikipedia
- http://www.world-nuclear.org/nuclear-basics/what-is-radiation.aspx
- http://www.ansto.gov.au/NuclearFacts/Whatisradiation/index.htm
- Letters from the country and abroad.
Hi @masudrana. Great article. I have a few suggestions though.
This is not a proper way of citing references. You need to provide links so that any of your readers who are interested can refer to the original article. Same goes for the images' source. I've made an article regarding this topic before. You can click here to find out.
This article is too long. Try to squeeze the information within 1000-2000 tops. It's difficult to focus on a lengthy article.
Please note that I'm not in any way, try to disapprove your work. You've done an excellent job here, but you can make it better. If you have any problem regarding STEM-related articles, you can join steemSTEM Discord Channel and we will be glad to assist you.
Thank you for you suggestion.I will try to do that next time! Reference edited though.
The Wikipedia link is broken.
@conficker its working fine with me.pls chack again.
Oh, my bad. Nice article btw. Good job.
Thank you @conficker
You got a 4.84% upvote from @brupvoter courtesy of @masudrana!
Congratulations @masudrana! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOPমাসুদ ভাই আশাকরি ভাল আছেন।
ভাই আমি steemit এ নতুন এসেছি। এ বিষয়ে সেরকম কোন ধারনা নেই। ভাই আপনার কি কোন গ্রুপ আছে। থাকলে আমাকে কি এড করা যাবে।
Amr kono group troup nai.so add korar o kono option nai😞😞😞😞 aka akai😩😩