Thermodynamic Balance And The Famous Triple Point Of The Mixtures
Hello friends of steemit today I want to continue sharing with you a little of the thermodynamic matter that I am so passionate about, the importance of this in any physical or chemical study of our lives.
When the variables necessary to describe the system have been specified, the state of the system has been specified. A system is in a defined state when all its properties have specific values. If, in turn, these values do not change with time, the system is said to be in thermodynamic equilibrium, for which there is no mass or energy flow. Thermodynamic equilibrium is established once the system reaches another. In thermodynamics, it is said that a system is in a state of thermodynamic equilibrium if it is unable to spontaneously experience any change of state when it is subjected to certain boundary conditions (the conditions imposed by its surroundings). Now, almost all the moderately complex and interesting systems that we find in practice are not in equilibrium. At the time, great minds of physics were raised whether it would be possible to extend the concept of thermodynamic equilibrium and, with it, the thermodynamic study itself, to more general systems. They found a generalization the sea of simple and, at the same time, applicable to almost every system that one could find. The thermodynamic properties of a system are given by the observable macroscopic physical attributes of the system, by direct observation or by some measuring instrument. A system is in thermodynamic equilibrium when no change in its thermodynamic properties is observed over time. The thermodynamic equilibrium state is characterized by the cancellation by compensation of exchange flows and the spatial homogeneity of the parameters that characterize the system that no longer depends on time. In vapor-liquid (VLE) is highly efficient in the separation and purification process.
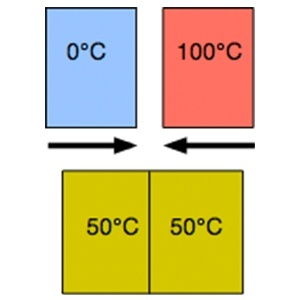
Diagram Phase
it is a diagram in which the variation between the different states of matter is plotted against certain given variables. The simplest phase diagram uses two variables, which is why it is also known as a 2D phase diagram (two dimensions). The two most commonly used variables are pressure and temperature. In a phase diagram of this type, corresponding to a pure substance, three zones can be distinguished in which the substance exists in a single state: solid, liquid or gaseous. There is a point at which the intersection of the three zones occurs, and this point is known as the triple point.
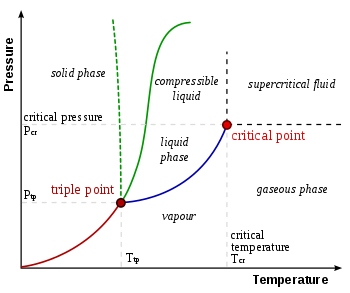
Between the separation of two zones a line is formed that represents pairs of pressure and temperature in which the substance passes from one state to another. For example, the points defined by pressure and temperature values at which a liquid passes to a solid state form the freezing line, represented in the previous image with the green solid line. The green dotted line is characteristic of water, which shows a different behavior to most liquids due to hydrogen bridges. Thus, if the pressure to which a liquid is subjected is known, it is possible to know the temperature at which it will solidify. In a similar way, the vaporization line is formed, between the liquid and gaseous state, and the sublimation line, which represents the direct passage from the solid state to the gaseous state without passing through the liquid phase. The triple point, is a point that is formed by the intersection of the three lines, the freezing, the vaporization and the sublimation. This means that in the triple point the matter coexists in the three states.
Properties of liquid phase from VLE data
Fugacity
Fugacity (f) is a measure of the tendency of a substance to escape from a phase in which it exists due to effects or influences of some chemical process. We can also see the fugacity of a gas as the effective pressure exerted by the gas.
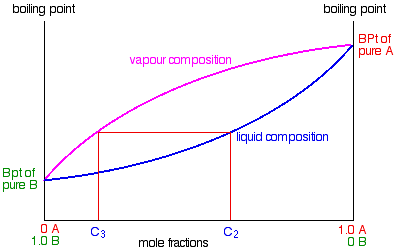
The figure shows a vessel in which a mixture of vapor and a liquid solution in equilibrium liquid vapor coexist. The temperature T and the pressure P are uniform throughout the vessel and can be measured with the appropriate instruments. Samples of the vapor and liquid phases can be extracted for analysis, and this provides experimental values for the mole fractions in the vapor {yi) and the mole fractions in the liquid {xi}.
Activity Coefficient
An activity coefficient is a number that expresses the chemical activity factor of a substance in its molar concentration. It is usually designated with the Greek letter γ (gamma). The measured concentration of a substance can not be an accurate indicator of its chemical efficacy as represented by the equation of a particular reaction; in certain cases, the activity is calculated by multiplying the concentration by the activity coefficient. In solutes, the activity coefficient is a measure of how much the solution differs from an ideal solution. The coefficient of activity of a species in solution is simply the ratio of its actual fugacity to the value given by the Lewis / Randall rule, to the same T, P, and composition.
Properties of activity coefficients
- The coefficient of activity of a chemical species is a measure of the effectiveness with which this species influences the equilibrium in which it participates. In very diluted solutions where the ionic strength is minimal, this effectiveness becomes constant and the activity coefficient acquires the value of the unit.
- As the ionic strength increases, the ion loses some effectiveness and its activity coefficient decreases.
- In low concentration solutions, the activity coefficient for a given species is independent of the nature of the electrolyte and only depends on the ionic strength.
- For a given ionic strength, the increase in the charge of an ion causes its activity coefficient to move away from the value of the unit.
- The activity coefficient of an uncharged molecule tends to unity, regardless of the ionic strength of the solution.
For more information consult the bibliography or links that I leave here:
Thanks for your constant support