Basic concepts to understand a chemical reaction
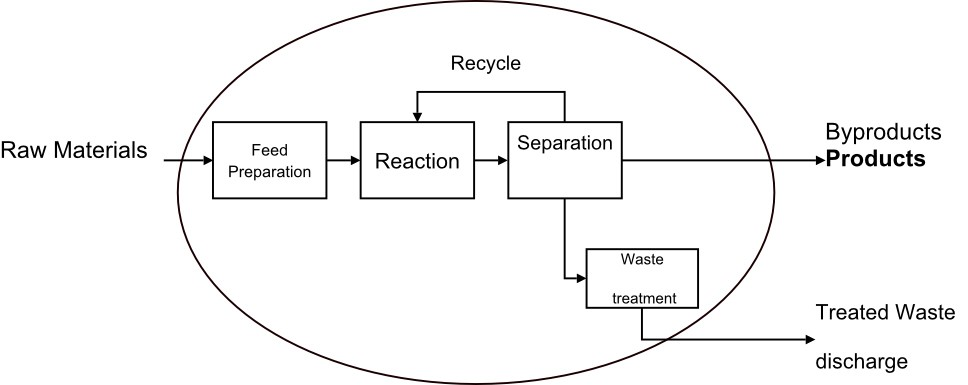
The chemical-industrial processes are designed to economically obtain a product from various unprocessed materials that are subjected to the different stages of treatment, as illustrated in image 1. Raw materials go through a series of physical treatments to Prepare them so they can react chemically, and then go to the rector. The products of the reaction must then undergo new physical treatments (separations, purifications, etc.) just get the desired final product. The design of the equipment for the stages of physical treatment is studied when treating unit operations. The interest of this post focuses on the stage of chemical treatment of a process. From the economic point of view, it is possible that this stage lacks importance since it could only consist of a mixing tank. However, often the chemical treatment stage is the core part of the process and that which makes or prevents the process from being economical. The design of reactors is not a routine task since for the same process it is possible to propose several solutions. In the search for the optimal design, it is not only the cost of the reactor that must be reduced to a minimum. It is likely that in a particular design the cost of the reactor will be low, but the materials that came out of the unit could do so in such a state that their treatment is much more expensive than in other designs. In this way. It is necessary to consider the economic aspects of the process in its entirety. In the design of reactors, the information, knowledge, and experience of several fields are used: thermodynamics, chemical kinetics, fluid mechanics, heat transfer, mass transfer, and economy. The engineering of chemical reactions is the synthesis of all these factors with the purpose of designing the best chemical reactor. In order to find out what a reactor is capable of, we need to know about the kinetics, the contact model, and the design equation.
Classification of reactions
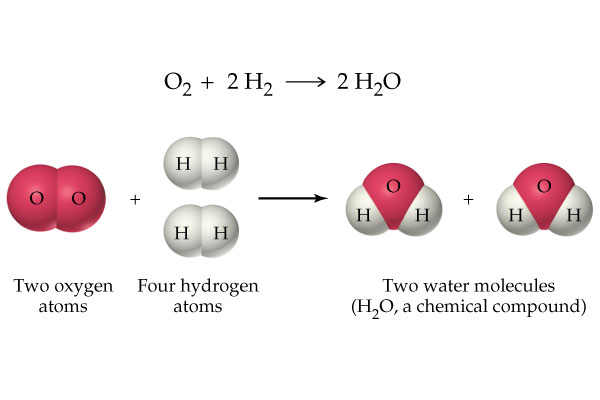
There are many ways to classify chemical reactions. In the engineering of chemical reactions. Probably the most useful method is to divide them according to the number and type of phases involved, resulting in two large groups: homogeneous systems and heterogeneous systems. A reaction is homogeneous if it is carried out in a single phase. heterogeneous if it is to be carried out - at the speed at which it is done. the presence of at least two phases is required. It is irrelevant whether the reaction is carried out in one, two or more phases or an interface, or if the reactants and products are distributed among the phases or are all in only one of them; what matters is that at least two phases are required for the reaction to be carried out in the way it does. An example in which the distinction between these systems is not clear is the group of very fast reactions. like the flame of the combustion of a gas. In this case, there is no homogeneity in terms of composition and temperature. Therefore, strictly speaking, there is not a single phase. since a phase implies uniformity with respect to temperature, pressure, and composition. The answer to how to classify these undefined cases is simple: It depends on how you choose to consider them. which in turn depends on which description is considered the most useful. In this way. only in the context of a particular situation is it possible to decide what is the best way to treat these cases undefined.
The reaction speed
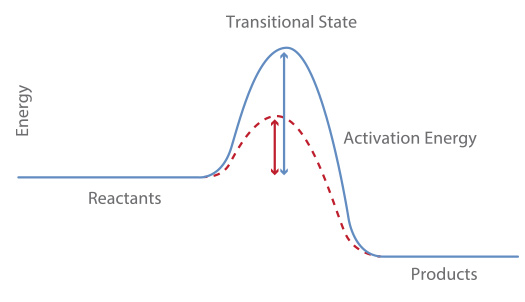
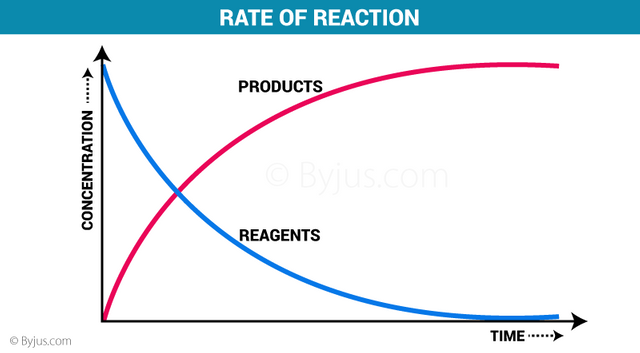
The study of the speed of a reaction and the factors that affect it belongs to an area of chemistry called chemical kinetics. The rapidity of a chemical reaction refers to the number of reactants that are consumed or products that are formed at a given time. The speed of a reaction can be understood with the theory of collisions, which tries to explain how substances react to form a product. This theory considers that the particles of the reactants have to collide effectively in order to react. An effective collision occurs when the particles have the proper orientation and collide with sufficient energy; it involves an energetic process and a defined arrangement of the reactant particles with each other.
Factors that affect the speed of the reaction.
The temperature of the reaction The temperature has a marked effect on the speed of reaction, which is seen in many situations of daily life. The rapidity of most chemical reactions increases when an increase in temperature occurs, and as a consequence, some reactions become violent and explosive. Although the rate of reaction does not increase uniformly with the increase in temperature, there is a general rule that is frequently applied in many reactions: the reaction rate doubles with the increase of every 10 T. However, there are reactions that become slower when the temperature increases. The increase in the speed of reaction by temperature can be understood in a simple way according to the theory of collisions, On the other hand, the increase in temperature also allows the particles to reach the activation energy faster, the minimum energy for react.
The presence of catalysts
Catalysts are substances that modify the speed of a chemical reaction without being consumed or suffering a chemical alteration that diminishes its capacity for subsequent action. There are two types of catalysts: the positive catalysts, which accelerate the speed of a reaction and the negative catalysts, which decrease it. In general, the term catalyst is commonly used to refer to a substance that accelerates the rate of reaction. Now, there are substances called inhibitors that can cause a catalyst to decrease its ability to accelerate a reaction. A catalyst acts to provide a different and easier path through which the reaction can pass and makes the reaction faster. The function of the catalyst is to lower the activation energy of the reaction, and thus allow a greater number of reactants to more easily reach the minimum energy to react, which increases the speed of the reaction in comparison with the uncatalyzed one.