Wave-particle duality
In the search for answers to our questions about the why and how of natural phenomena, we often find that they are explained by postulates or physical laws and complex mathematical expressions that escape our understanding, so we abandon our interest in finding that answer that satisfies our curiosity. As a Physics student, I have run into challenges typical of every career, maybe my curiosity has gone further. Currently there is a large amount of teaching resources, as is this medium, which makes our understanding easier, so I invite you to don’t be discouraged in that search for knowledge.
The topic that I share with you today is about the fascinating discovery of wave-particle duality.
The science that studies and explains this behavior is quantum mechanics, which largely is based on mathematical models that for many are abstract and complex because they have their own mathematical language that differentiates it to a great extent from that used in classical physics. However, the creation of its postulates and mathematical models arose from the need to explain phenomena that are not so difficult to understand. In this presentation I hope to be able to make you understand their content, without the need to have deep knowledge of physics.
The understanding that we currently have of the behavior of the universe has been thanks to the work of great scientists who laid the theoretical and experimental bases that have given clarity to the microscopic physical phenomena that classical physics could not explain and that for years we didn't know.
The structure of the atom and how electrons orbit, gave rise to postulates and models, some successful and others erroneous or incomplete, that allowed to advance in the formulation of new theories whose applications at present have been translated into the benefit of humanity.
The photoelectric effect realized by Albert Einstein was one of the phenomena in which the physics as it was known up to that moment did not give a clear explanation of the obtained results. The detachment of electrons from a material when a wave of light with a certain frequency impacts on it, led to the fact that the light must be constituted by particles called Photons, whose energy and linear momentum (amount of movement) are given by E = hf and p = h / λ respectively, where "h" is a constant known as the Planck constant, "f" the frequency and "λ" the wavelength.
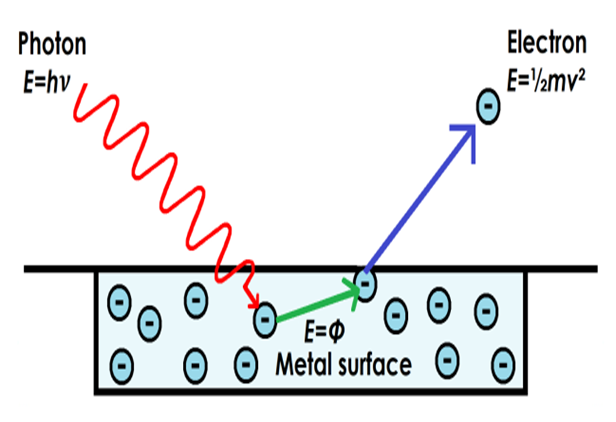
Source
Another fact that validated that a wave is made up of particles was the dispersion of X-rays made by Arthur Comptom. In the figure 2, the X-rays are represented by the incident photon, where its collision with an electron at rest generates a deviation of the incident X-rays. The deviations obtained according to the model proposed by Compton coincided with the experimental measurements, thus confirming the validity of the corpuscular treatment of the wave.
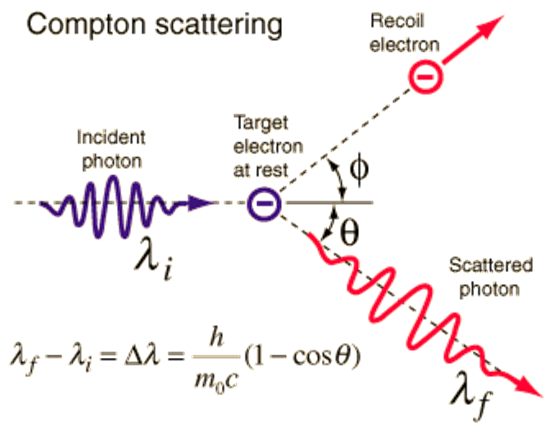
Source
These two events ended up being decisive for the scientific community that until then was skeptical of such assertions.
Now, if light or X-rays are waves and a great variety of experiments such as those made by Thomas Young show that light waves are diffracted due to their unequivocally undulatory property, how can they be a particle?
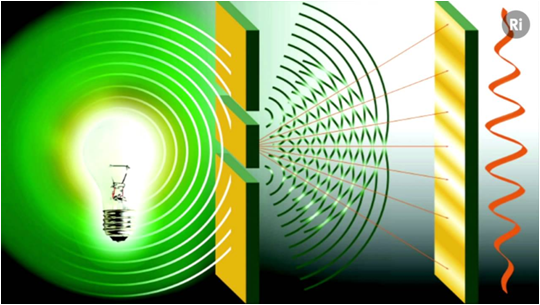
Source
The consensus was to accept the duality of light.
"Light has a dual nature: it shows both wave and particle characteristics, its treatment will depend on the observed phenomenon"
The already solved problem of duality gave rise to another question. In this case the protagonist was Louis De Broglie who raised the possibility that material particles such as electrons had a wave behavior.What De Broglie proposed was a duality now seen in the other direction from particle to wave. His approach was controversial as many in the history of physics. According to De Broglie the relation (λ = h / p) should be valid for material particles.
The connection between a descriptive parameter of a particle as the linear moment and a proper parameter of the waves "λ" was a transcendental fact that showed the duality of matter.
The experimental confirmation of the theorized by De Broglie was immediate. If this theory was correct, the electrons, like the waves, would show diffraction patterns.
This was verified by G. P Thomson who obtained circular patterns of diffraction when an electron beam passes through a crystal as illustrated in figure 4.
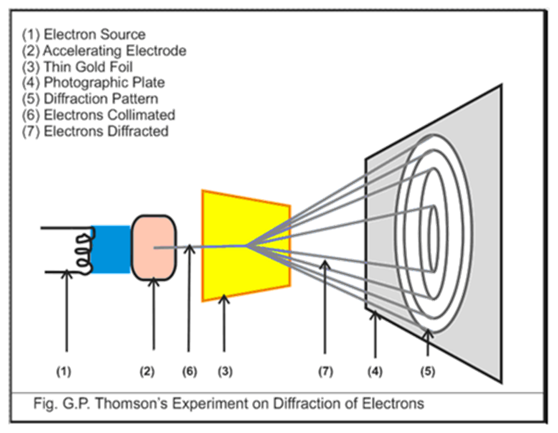
Source
Another of the experiments that confirmed what De Broglie predicted was that carried out by the scientists Davisson and Germer (See figure 5), where the wavelengths obtained experimentally from the diffraction angles of an electron beam, were consistent with those obtained from his equation.
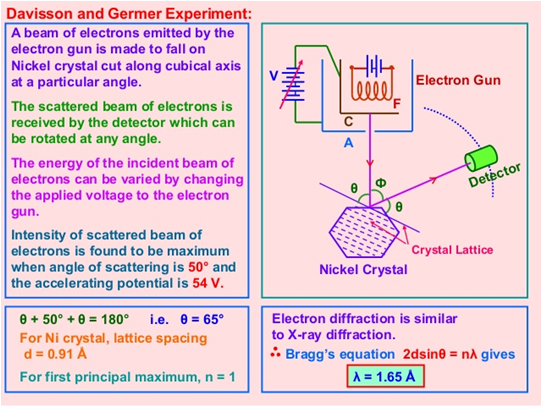
Source
In the above it is important to appreciate that both the energy (E = hf) proposed in the photoelectric effect and the de Broglie wavelength (λ = h / p), are mathematically simple expressions, but with a deep physical content that frames the beginning of the quantum era and served as the basis for future mathematical and physical models that to this day support quantum mechanics.
I hope I have achieved my goal of sharing this information in a simple and easy to understand way. If you have any comments and / or contributions that you want to make to it, they will be well received.
Until a next opportunity.
References
Physics editorial Addison-Wesley / Marcelo Alonso, Edward J. Finn
Physics for Scientists and Engineers, Vol.2 Fifth Edition / Paul A. Tipler , Gene P. Mosca
Fundamental University Physics, Vol. 3: Quantum and Statistical Physics / Marcelo Alonso, Edward J. Finn
Fundamentals of Physics. Halliday & Resnick. Jearl Walker.10th. Edition. Wiley editorial.