Chemistry and Metabolism of Biomolecules #6: Chemistry of Proteins
Introduction
Proteins are basically polymers of amino acids. They are a very vital component in biological systems. As a matter of fact, most biological processes are carried out by proteins in the form of enzymes, hormones and a lot of chemical messengers.
Proteins are synthesized through a process known as translation. This is the last step in the central dogma of molecular biology. It basically involves the conversion of an mRNA (messenger RNA) into protein.
This process is quite complex as it involves a lot of enzymes as well as several complexes and the movement of substrates within enzyme subunits. I'll spare you the details for now.
Proteins are basically composed of many units of amino acids held together in peptide bonds. A peptide bond is formed when the ~COOH group of one amino acid is linked to the ~NH2 group of another molecule giving off a water molecule.
Illustratively, it's a lot like this
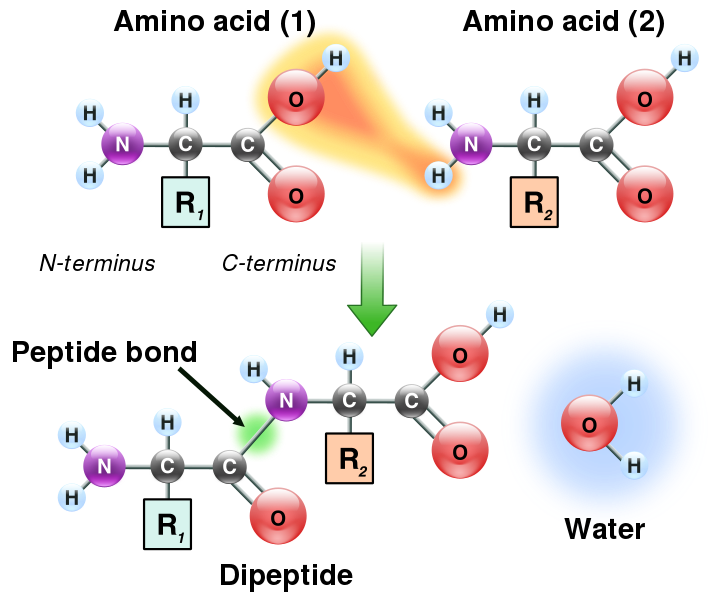 How Peptide Bonds are Formed (License: Public Domain]:
Wikipedia
How Peptide Bonds are Formed (License: Public Domain]:
Wikipedia The amino acids are continually joined in peptide bonds in the process of forming a protein molecule.The order in which amino acids are arranged in proteins is determined by information stored in the DNA template that leads to the formation of this protein.
A Brief Look at the Structural Levels of Organization of Proteins
Just like the carbohydrates, the proteins are arranged in different levels structurally but they are quite outlined and defined differently.
- The Primary Structure
- The Secondary Structure
- The Tertiary Structure
- The Quartenary Structure
The Primary Structure
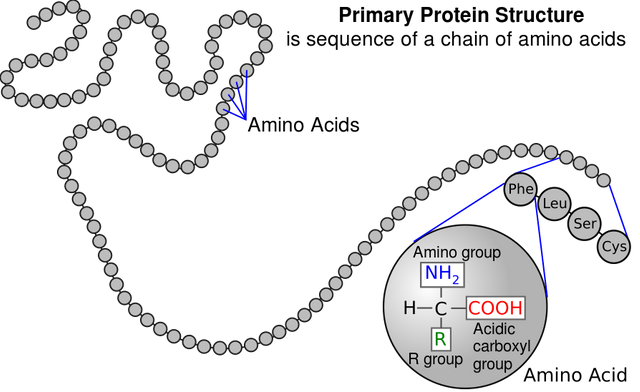 The Primary Structure of a Protein is the sequence of amino acids (License: Public Domain]:
Wikicommons
The Primary Structure of a Protein is the sequence of amino acids (License: Public Domain]:
Wikicommons The primary structure of a protein is basically made up of the amino acid sequence. It determines the function of a protein. Changes to the primary structure of a protein affects the overall function of that protein.
A case where changes in the primary structure of a protein can affect a protein's function is in sickle cell anemia. Here, glutamate at position 6 is replaced by valine on the B chain of haemoglobin, a protein (Haemoglobin has an A and a B chain).
The primary structure is almost the most stable structure of the protein. It is not affected by denaturation.
The Secondary Structure
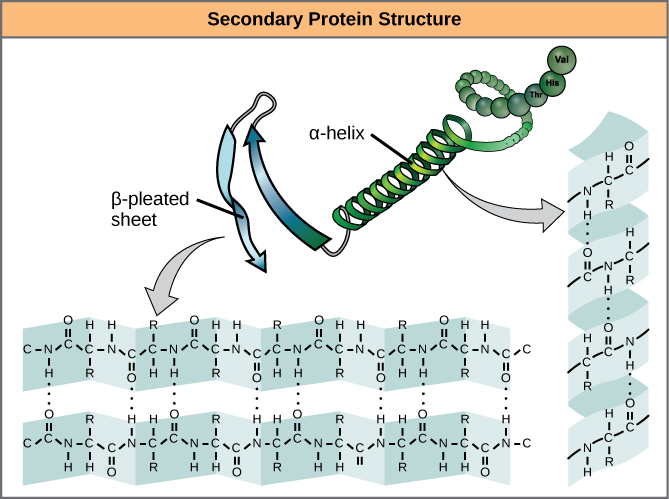 The secondary structure of a protein comprises the alpha helix and the beta-pleated sheet (License: CC-BY 4.0, Author: OpenStax]:
Wikicommons
The secondary structure of a protein comprises the alpha helix and the beta-pleated sheet (License: CC-BY 4.0, Author: OpenStax]:
Wikicommons The secondary structure of proteins is quite different and more complex than the primary structure. Amino acids within the polypeptide chain interact with each other leading to the formation of a 3D structure. In this case, the amino acids are held by hydrogen bonds and not the usual peptide linkages.
The secondary structure could be in the form of an alpha helix or a beta-pleated sheet. The major difference is in how the hydrogen bonds are formed.
The alpha helices are in coily forms just as you would picture a typical helix. In this structure, the NH and C=O portions of amino acids on a single polypeptide chain interact leading to the formation of hydrogen bonds.Thus, there is intrachain hydrogen bonding here. The alpha helix possesses a spiral structure, thus, it is arranged in turns.
Each turn of an alpha helix is made up of 3.6 amino acid residues and the average distance between two amino acid residue is 1.5 Å. The alpha helix is also right handed just like the DNA double helix.
In the beta-pleated sheets, hydrogen bonds are formed between C=O and NH groups of adjacent polypeptide chains. Thus, there is interchain hydrogen bonding here. The beta-pleated sheets have chains running either in parallel or anti-parallel direction to each other. This depends on how the carboxyl (COOH) and amino (NH2) terminal groups are situated on both chains.
The Tertiary Structure
 The tertiary structure shows the superfolding of a protein molecule (License: Public Domain]:
Wikicommons
The tertiary structure shows the superfolding of a protein molecule (License: Public Domain]:
Wikicommons This structure results when either the alpha helix or beta-pleated sheets fold and twist about itself. This results in amino acids which would otherwise be far apart interacting with each other due to the folding. The tertiary structure is the practical representation of the three dimensional structure of a protein.
Hydrogen bonds, disulfide bonds, electrostatic interactions and Van der waals forces are the forces that keep the amino acids present in this structure in interaction.
The Quartenary Structure
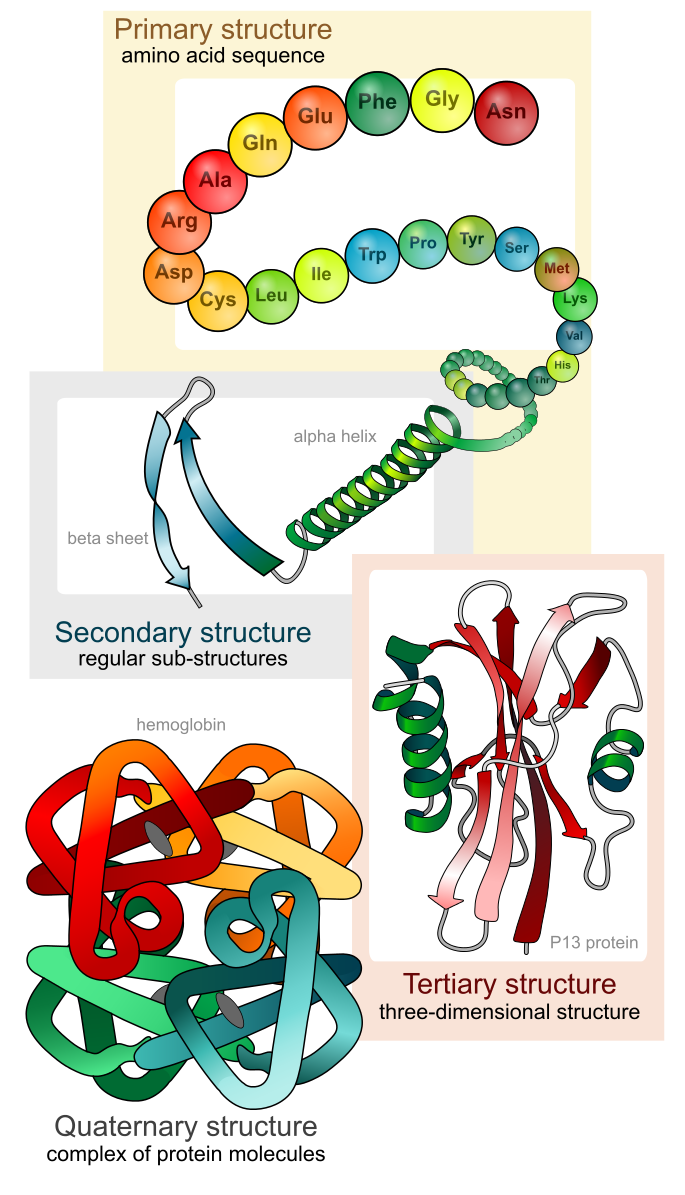 The Quartenary Structure is made up of more than two polypeptides interacting to form a superfolded Structure (License: Public Domain]:
Wikicommons
The Quartenary Structure is made up of more than two polypeptides interacting to form a superfolded Structure (License: Public Domain]:
Wikicommons This arises when two or more polypeptide chains interact by non-covalent bonding. A normal protein is usually made up of one polypeptide chain (monomer) but in this structure, dimers (proteins with two polypeptide chains), trimers (three polypeptide chains) and tetramers (four polypeptide chains) can arise. Haemoglobin A is a tetramer as it contains two alpha chains and two beta chains.
The Structure of a Protein is relevant for its Function
The structure of a protein is very important for its functioning. Alteration in the structure of a protein can have a profound effect on its function.
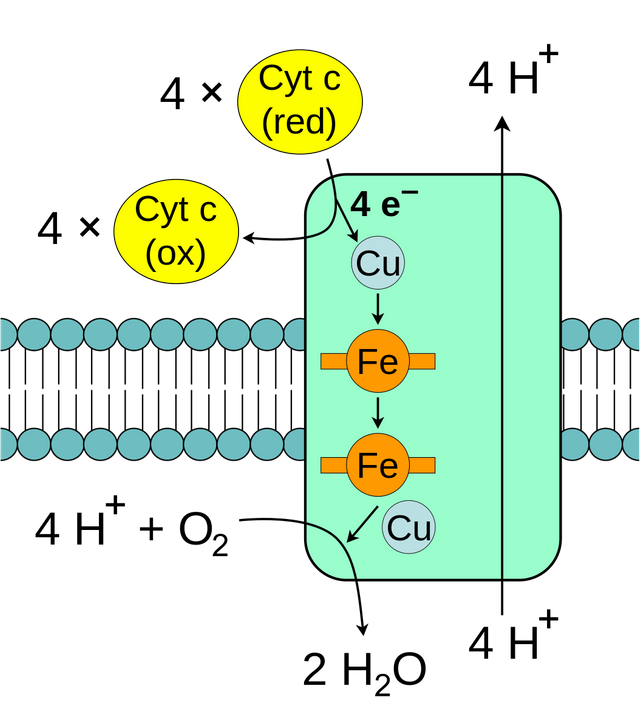 Cytochrome c oxidase is a metalloprotein requiring copper and iron for its action (License: Public Domain]:
Wikicommons
Cytochrome c oxidase is a metalloprotein requiring copper and iron for its action (License: Public Domain]:
Wikicommons Enzymes are made up of active sites (binding surfaces or clefts for substrates). The active site of an enzyme is usually biologically designed in a way that favours the binding of specific substrates. Some enzymes also contain co-factors like metals (Zn2+ is a co-factor seen in carbonic anhydrase). Enzymes which contain metals as a prothestic group are known as metalloproteins. Any alteration in the active site of a metalloprotein, say the absence of its prothestic group will alter its function rendering it incapable of catalysis.
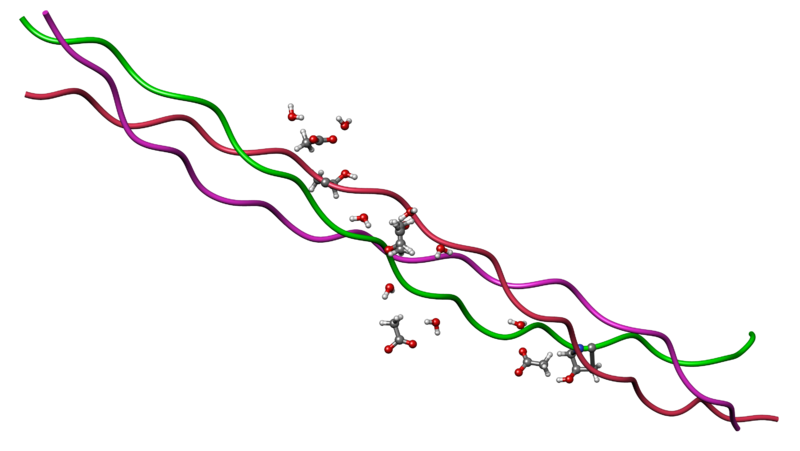 The triple helical structure of collagen (License: Public Domain]:
Wikipedia Commons
The triple helical structure of collagen (License: Public Domain]:
Wikipedia Commons Another case of structure determining the function of a protein can be seen in collagen. Collagen is a triple helical structure consisting predominantly of glycine. Constant repulsion bettwen the hydroxyproline molecules as well as hydrogen bonds is responsible for the stability of this structure. In Vitamin C deficiency, hydroxylation of proline to give hydroxyproline is greatly reduced and thus the collagen structure is weakened. This can cause tissues of the skin to become fragile and possibly break resulting in bleeding in some cases. This is basically the mechanism behind scurvy.
The last case in seen in haemoglobin which i have highlighted above. There are a lot of cases in nature but these three give succint illustrations of how structures of proteins are relevant for their functions.
Classification of Proteins
The classification of proteins has proven to be quite a difficult task but biochemists generally came to a consensus and the following criteria for classification were mapped out.
- Based on function
- Based on composition
- Based on size and shape
Classification Based on Function
The proteins have a wide range of functions in the biological system and this has formed a basis for their classification. Taking a holistic approach at this classification, there are numerous sub-classifications in which proteins are listed.
Enzymes are grouped under catalytic proteins as their main function is catalysis. Immunoglobulins are defence proteins as they are mainly involved in immunity which is a form of defense. Myosin and Actin are contractile proteins as they are necessary for muscle contraction. Haemoglobin is a transport protein as it transports oxygen and carbon dioxide in the body. Hormones are generally considered regulatory proteins cause they regulate biological processes in the body. Histones are considered genetic proteins because they interact with genetic material like the DNA.
Classification based on Composition.
Here, we have the simple proteins, the conjugated proteins and the derived proteins.
The simple proteins are structurally composed of just amino acids. Example of the simple proteins include; Albumin, histones, lectins, globulins and scleroproteins.
The conjugated proteins as the name would imply are usually joined to a prosthetic group (the non-protein part). The protein part is generally referred to as the apoprotein while the whole compound (apoprotein + prosthetic group) is collectively referred to as the holoprotein. Examples of the conjugated proteins include: the metalloproteins, the nucleoproteins (contains nucleic acid as the prosthetic group), mucoproteins (contains mucopolysaccharides as the prosthetic group), glycoproteins (contains carbohydrates) and lipoproteins (contains lipids) e.t.c.
The derived proteins are gotten from the simple and compound proteins. When these simple and compound proteins are degraded, the resulting structure is a derived protein. Examples include: Myosan gotten from myosin, Fibrin gotten from fibrinogen, chymotrypsin gotten form chymotrypsinogen etc.
Classification based on Size and shape.
They can be divided into fibrous and globular proteins here.
The fibrous proteins are elongated in shape and very difficult to digest. They are also relatively large in size. An example is collagen.
The globular proteins are oval in shape and quite easy to digest. They are relatively smaller in size. An example is albumin.
Summary
The proteins are very diverse class of biomolecules. It has been established that proteins are polymers of amino acids. They play very essential roles in biological systems and their classification based on function has buttressed that. I hope you learned something new today. Watch out for the chemistry of nucleic acids. Till next time.
References
Proteins. Wikipedia articles (accessed on April 29th, 2018)
Chatterjea, M. N. (2012). Textbook of Medical Biochemistry. (8th Edition). Jaypee Brothers Medical Publishers. New Delhi. India. pp. 83 - 94.
Vasudevan et al. (2011). Textbook of Biochemistry. (6th Edition). Jaypee Brothers Medical Publishers. New Delhi. India. pp. 27-39.
Image Sources
All images are from pixabay and wikicommons licensed under creative commons and eligible for commercial use.
I'm a proud member of the steemstem community which promotes quality posts in the science, technology, engineering and mathematics fields on the steem blockchain mainly through interaction and engagement. Feel free to join us on discord here
Is it accurate to consider the secondary structure of proteins as a 2 dimensions model representation ? Am I missing something here ?
LOL the secondary structure is actually a 3D representation of a protein but the tertiary structure is a much more perfect representation.
No, it is not a 2D representation of protein. I personally feel the primary structure would pass for that but I haven’t seen that anywhere so I can’t particularly verify that :)
Okii got it :)
Primary structure is 1D (if you mean sequence info). And if you just consider a poly-peptide, its again 3D
That’s true. Makes a lot of sense that way.
This is a new line for me, i learnt something even though I struggled with some terms. So, what type of protein is found in the food we eat? beans for example
Simple proteins
Oh... alright, thanks
Much of this is well over my head, but I learned a great deal regardless. Thanks for sharing.