Chemistry and Metabolism of Biomolecules #4: Chemistry of Lipids
Introduction
So we’ve gone quite far with this series and we’ve just concluded the chemistry of carbohydrates. The metabolism of carbohydrates which you will appreciate more is coming much later.
A new biomolecule is introduced in this installment of this series. The lipids.
Lipids are generally molecules of biological origin made up of fatty acid esters, alcohol (not in all cases) and in some cases, phosphoric acid, carbohydrates and nitrogenous bases. They are insoluble in water but soluble in polar solvents like ether, chloroform and acetone.
They are the primary source of energy for the body and if you began this series with me, you’ll remember when I mentioned glucose as the secondary source of energy and asked what you thought the primary source of energy for the body was. Well, that’s no brainer now as you have found that out here.
Classification of lipids
The classifications of lipids are quite numerous but a general classification has been accepted. This is the The Bloor’s Classification of Lipids
In 1943, Bloor classified lipids into four categories based on a particular set of criteria. These criteria include;
- A lipid must be insoluble in water
- Must be soluble in one or more organic solvents like ether, chloroform or benzene.
- A lipid must have some similarities with the fatty acids
- Must be easily utilized by living organisms
These criteria formed the basis for bloor’s Classification of lipids. So according to Bloor, lipids can be generally classified into;
- Simple lipids
- Compound lipids
- Derived lipids
Simple Lipids
The simple lipids in the simplest terms are the Lipids that just consist of fatty acids attached to an alcohol molecule which serves as the backbone for the entire structure. They have no other molecules attached to them.
The simple lipids comprises the fats and waxes generally.
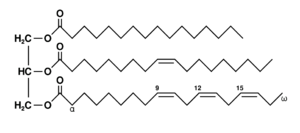
Fats are simple lipids that consist of fatty acids attached to glycerol. They are also called Triacylglycerols or Triglycerides. Breaking down this alternative name for fats, you’ll see that it implies that fats contain three fatty acids esterified to a glycerol molecule (Tri = 3, acyl = a general connotation for fatty acids, glycerol = an alcohol).
When one hears of fats the next thing that comes to mind is “oils”. Well, oils are just simply fats in a liquid state. Yep, fats can become oils and oils can become fats. It just depends on what the temperature is.
Waxes, on the other hand, consist of long chains of fatty acids attached to an alcohol molecule. In fats, the fatty acids have a short chain but the opposite is the case in waxes. An example of waxes is the beeswax produced by honest bees.
Compound Lipids
The compound lipids contain in addition to their fatty acid esters, other compounds attached to their alcohol backbone too. These compounds range from carbohydrates to phosphate and sulfate groups. They can be sub-classified into two groups based on the additional groups they contain.
- The phospholipids
- The non-phosphorylated lipids
The phospholipids are generally composed of the glycerophosphatidates (both nitrogen and non-nitrogen containing), the plasmalogens and the phosphosphingosides.
The non-phosphorylated lipids are composed of the glycosphingolipids and the sulpholipids.
Phospholipids
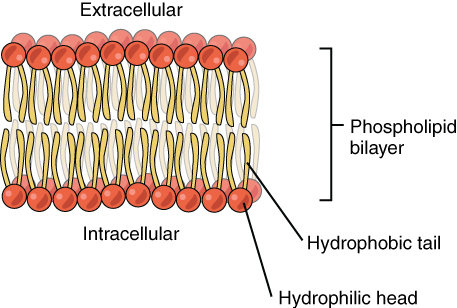
From our little knowledge of cell biology, we would know by now that phospholipids are an integral part of the biological membrane. They are amphipathic molecules (they possess polar and non-polar ends) and can form orientations at oil-water interfaces. This means their polar ends get situated in water and their non-polar ends in oil.
The phospholipids are made up of glycerol, fatty acids and a nitrogenous base. Due to their amphipathic nature, they can form micelles and liposomes.
The phospholipids are further classified based on the type of alcohol they contain. With that being said, we have;
- Glycerophosphatides which contain glycerol as their alcohol backbone. E.g Lecithin, plasmalogens and cardiolipins.
- Phosphosphingosides which contain sphingosine as their alcohol backbone. E.g Sphingomyelin
- Phosphoinositides which contain inositol as their alcohol backbone. E.g. Phosphatidylinositol.
The glycerophosphatides are made up of the nitrogen containing (Lecithin, Cephalin and phosphatidyl serine) and the non-nitrogen containing glycerophosphatides (phosphatidyl inositol).
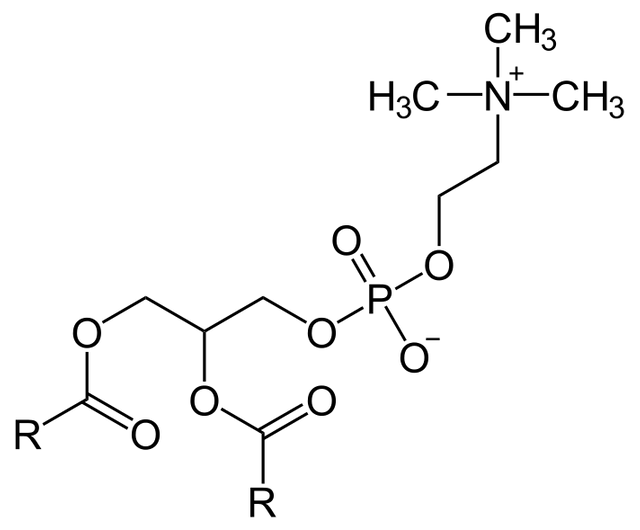
Lecithin is also known as Phosphatidyl choline. It has two fatty acids esterified at its alpha (first) and beta (second) carbons. At its third carbon, it has a phosphoric acid group to which is attached choline, a nitrogenous base.
All other phospholipids follow this arrangement, differing only in the alcohol they contain as well as in the nitrogenous base moiety.
Cephalin, otherwise known as, Phosphatidyl ethanolamine for example has ethanolamine as its nitrogenous base. Phosphatidyl serine has serine and Phosphatidyl inositol has inositol. I guess we get all get the story by now.
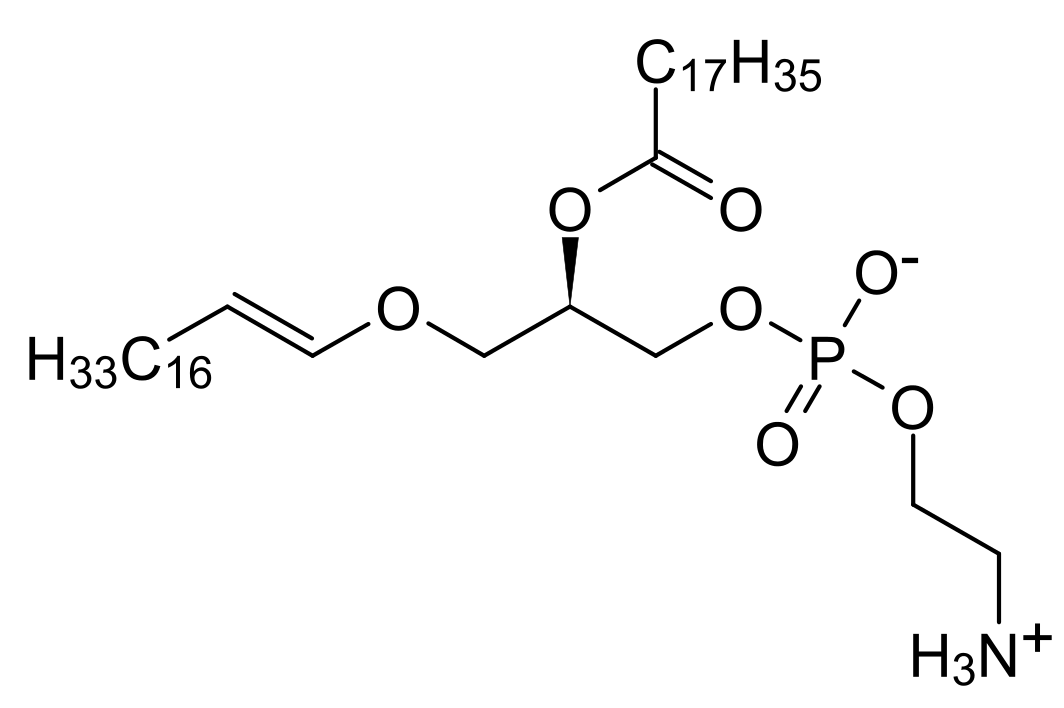
Plasmalogens however show a little diversion from this common arrangement. At its alpha carbon, it contains a long chain aliphatic aldehyde contained in ether linkage to the first OH group of the glycerol backbone. The other OH groups have a fatty acid and phosphoric acid linked to either ethanolamine or choline attached to them.
The phosphosphingosides have sphingosine as their backbone structure as I earlier stated. A notable example is sphingomyelin which is heavily found in the nervous system. Accumulation of the sphingomyelin molecules in the brain, liver and spleen leads to a condition known as Niemann-Pick Disease. There is an enzyme deficiency in this case. This enzyme is Sphingomyelinase which breaks down sphingomyelin to ceramide and phosphoryl choline.
The Non-Phosphorylated Lipids
This class of compound lipids do not contain phosphate grounds at any point. They are further subdivided into;
- The glycosphingolipids.
- The sulpholipids.
The glycosphingolipids are majorly made up of a carbohydrate moiety and ceramide. They include;
- Cerebrosides
- Gangliosides
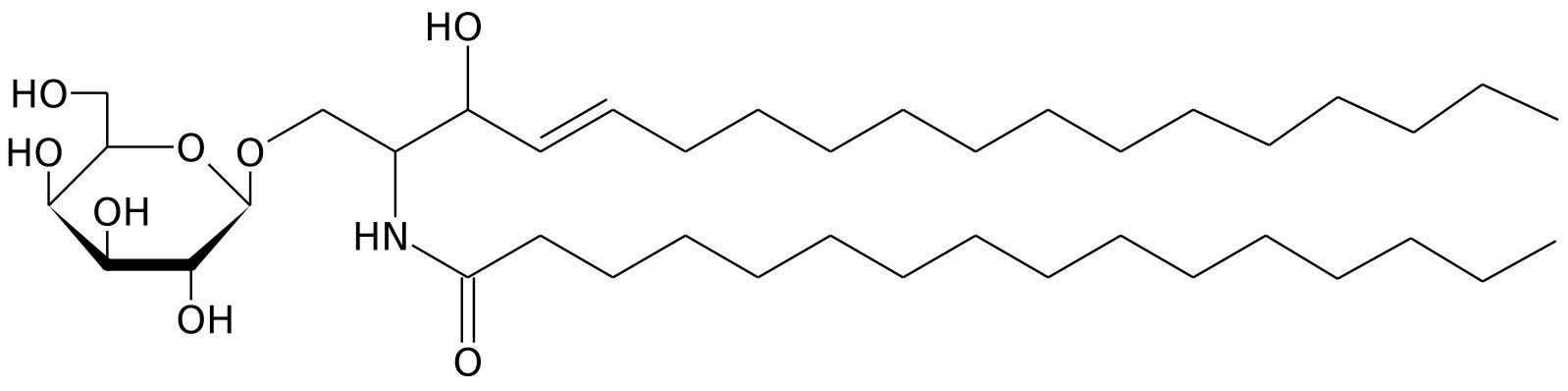
Cerebrosides are majorly found in the white matter of the brain. They do not contain a phosphate group, glycerol not a nitrogenous base. They yield a sugar molecule, a fatty acid and sphingosine on hydrolysis. The cerebrosides are of four types and this division is based on the type of fatty acids they contain
- Kerasin which contains lignoceric acid
- Cerebron which contains cerebronic acid
- Nervon which contains nervonic acid (cliche at this point).
- Oxynervon which contains a derivative of nervonic acid.
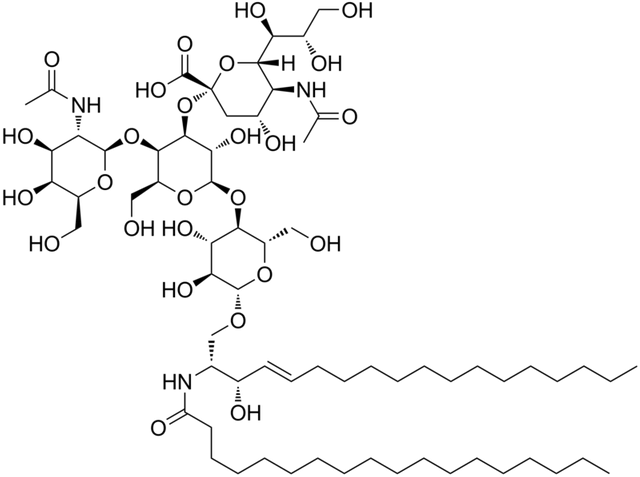
Gangliosides, on the other hand, are found predominantly in the grey matter of the brain. They have been considered the most complex glycosphingolipids known to man. They contain two hexose (six carbon sugar e.g glucose) or hexoamine (hexose + amino group e.g N-acetyl galactosamine) molecules attached to ceramide as well as NANA (N-acetyl neuraminic acid) as an integral part of the overall structure.
There are a lot of gangliosides in nature but the commonest and most important ones are; GM-1, GM-2, GM-3 and GD-3.
The gangliosides have been claimed to serve as binding sites for certain hormones and are thus, vital components of membranes.
The sulpholipids contain a sulphate group in their overall structure. They are believed to be Sulfate esters of glycolipids. They do not have specific nomenclatures. They are just observed to contain Sulfate groups.
Derived Lipids
The derived lipids are those which are gotten from the hydrolysis of the simple and compound lipids and still maintain the usual properties of lipids. They are largely grouped into;
- Fatty acids
- Monoglycerides and Diglycerides
- Alcohols
The fatty acids are inorganic acids which occur in triglycerides and range from 2 to 24 carbons in chain length. They can be classified based on a number of various properties but I’ll be looking at just two factors for the sake of convenience and easy comprehension.
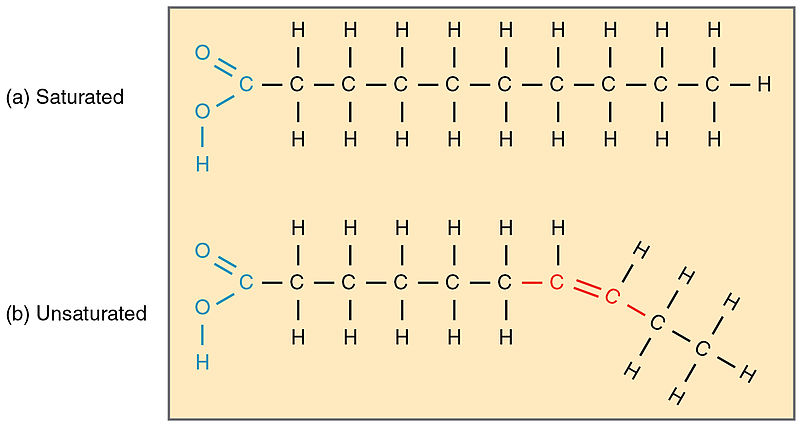
Fatty acids can be classified based on the presence or absence of double bonds in their structure into saturated and unsaturated fatty acids.
The saturated fatty acids do not contain double bonds. They have a general formula: CnH2n+1 COOH. They have the suffix -anoic in their nomenclatures. The most common saturated acids in nature are palmitic acid (with 16 carbons) and stearic acid (with 18 carbons). The aforementioned are most abundant in body fats.
The unsaturated fatty acids contain double bonds. They have a general formula: Cn H2n–1 COOH. They have the suffix -enoic. They can exist in cis and trans forms due to the presence of double bonds which causes rotations at their point of location. In biochemical terms, they exhibit geometrical isomerism.
It’s been observed that during the course of metabolism, the trans fatty acids are formed and the trans fatty acids have been noted to be injurious to health. The body however has a way of changing these trans fatty acids to cis fatty acids which is beneficial.
Examples of unsaturated fatty acids are oleic acid and linoleic acid.
Fatty acids can also be classified on based on nutritional requirement;
- Essential fatty acids
- Non essential fatty acids
The essential fatty acids cannot be produced by the body and so must be obtained in diet. The three main essential fatty acids are linoleic acid, linolenic acid and arachidonic acid. There’s a grey area in the regarding arachidonic acid as an essential fatty acid.
This is due to the fact that arachidonic acid can be synthesized from linoleic acid in a three stage reaction involving the addition of acetyl coA. However, due to the fact that the body is not capable of de novo synthesis of arachidonic acid, they get a pass and can belong here comfortably.
The monoglycerides and diglycerides contain one and two fatty acids attached to their ~OH group of their glycerol backbones respectively. They can be obtained from incomplete hydrolysis of triglycerides.
The alcohols obtained after hydrolysis can be glycerol or sphingosine.
However, there are derived lipids that can be gotten as products of metabolism and they include the sterols (e.g cholesterol), the terpenes, the leukotrienes, prostaglandins, carotenoids but this is not a topic I’ll want to delve into fully for the sake of sparing my readers with the biochemical mumbo jumbos. However, if you are interested in knowing more you can check out here for starters.
Summary
Lipids are the primary sources of energy for mammals. They are also starter molecules for the synthesis of vital hormones like prostaglandins as well as vitamins like Vitamin A. They are quite diverse, also of high molecular weights and very vital for existence. I hope you learned something today. See you on the next installment of this series: The chemistry of amino acids and proteins. You’ll appreciate this more. Thank you for coming around as usual.
References
Chatterjea, M. N. (2012). Textbook of Medical Biochemistry. (8th Edition). Jaypee Brothers Medical Publishers. New Delhi. India. pp. 45-64. Vasudevan et al. (2011). Textbook of Biochemistry. (6th Edition). Jaypee Brothers Medical Publishers. New Delhi. India. pp. 73-82. Image Sources I'm a proud member of the steemstem community which promotes quality posts in the science, technology, engineering and mathematics fields on the steem blockchain mainly through interaction and engagement. Feel free to join us on discord here
Lipids
Simple Lipid
Wax
All images are from wikicommons licensed under creative commons and eligible for commercial use.
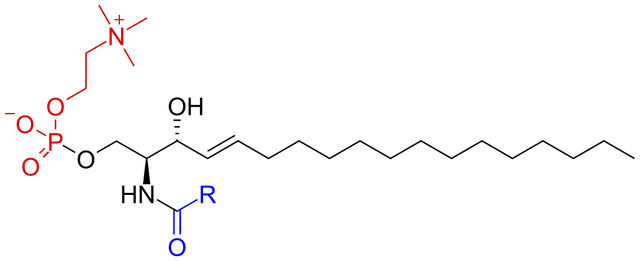

Biochemistry helps us to understand how living organisms utilize the chemical energy in their environment to carry out their biochemical activities.
This requires an understanding of the simpler principles of physical chemistry and thermodynamics as they apply to a living organism.
Nice read sir!
Thank you man
Welcome always
You are going good. I am not good in chemistry but still, your article is easy to understand.
Thank you, Kamesh. I’m glad you found it easy to digest
Super!
Thanks prof.
This post has been upvoted and picked by Daily Picked #35! Thank you for the cool and quality content. Keep going!
Don’t forget I’m not a robot. I explore, read, upvote and share manually ☺️
Thank you. Surely, I will !
Seeing this post was a good coincidence I think. I happen to be offering a Biochemistry course and we're currently on lipids. Reading it in my book seem a hard task but reading this post made it so Appetizing I'm gonna open my book right now 😁😁
That’s great man. Glad you found it helpful 😀
Keep the good work buddy. You're building something great with this series.
You're really an authority in this field.
Nice input (thanks for forcing those foreign words into my head )
Lol it’s my pleasure man. Thanks for dropping by once again.
Always a pleasure fam
Here again is another great effort from you. I see the idea behind this series. It really is a good one. People can just check through your blog and have themselves educated.
Nice work!
This is already a lecture room. Guess @kingabesh is preparing already
lol na so o
Go for it boss
Ah you’ve always been a man of vision, Temitayo. Thanks for coming around
My pleasure.
Many of the compounds you mentioned here( e. g choline and cephalin) I got to learn from studying my Scrabble lists, but in this post they came alive as I could see how useful they actually are in real life and how they are connected.
Also, the part about saturated and unsaturated fatty acids and the suffixes in their names, that I never knew. I can go on and on about the things I've learned here, such is the quality of this post. Kudos, or more appropriately KUDOSES.
Thanks man. I’m glad you could learn something new :)
Now I got another article which makes my life easy. :) I can refer my readers to this article for reading about phospholipids etc :)
Thanks man. Sure you always can :)
Congratulations @kingabesh! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOP