Glow Sticks? – Is it magic or what?!
Hi fellow Steemians! Today I want to talk about this amazing piece of technology that makes you a wizard. Kinda. Is there even anyone that does not love glow sticks? They are just great! You certainly went to some party where they handed them out, or even bought some by yourself. So, you bend it and pffft – it starts glowing! But why is that? How does it work? Hmm?
Let’s find out!
Is it magic?
No. Thank you for reading this article, have a great day!
Nah, gonna explain how it works, no worries. :D
And then he turned water to wi….eh..light!
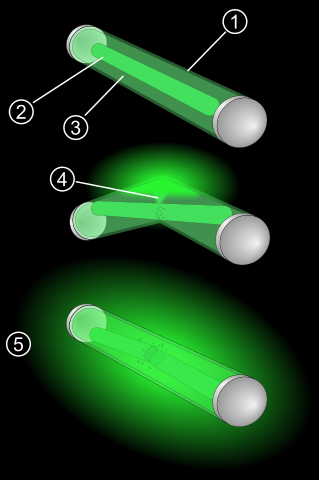
So, basically the emission of light is due to chemiluminescence (light produced by chemical reaction). If you take closer look at this glow stick you may notice that inside the plastic casing is another casing made from glass. Each of these tube casings (plastic one and glass one) holds different solution. And when you bend the glow stick the glass casing shatters and thus these two solutions mix. And this triggers a chemical reaction that produces light.
1. Plastic casing holds everything inside.
2. Inner glass vial holds Hydrogen Peroxide.
3. Diphenyl Oxalate and fluorescent dye solution.
4. Mixing of solutions triggers chemical reaction.
5. Which results in light emission.
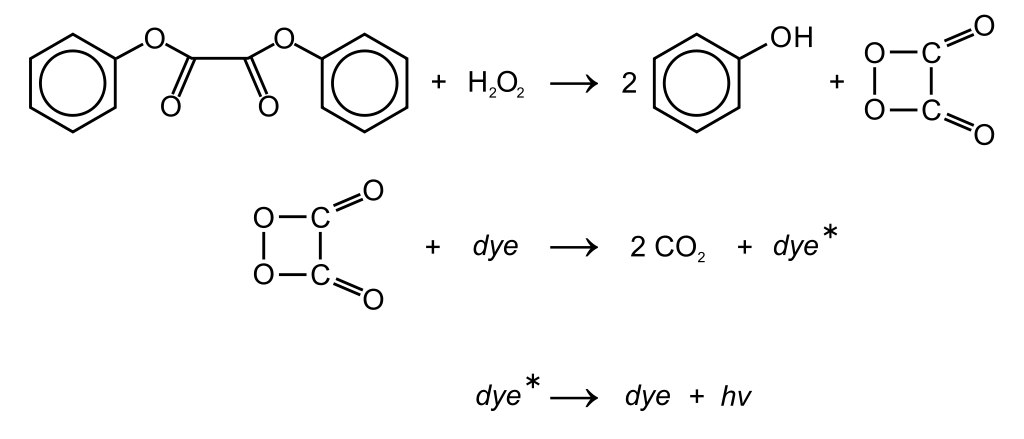
Now in more detail. The solution in plastic casing consists of dye and diphenyl oxalate. The glass casing is filled with hydrogen peroxide. When hydrogen peroxide and diphenyl oxalate mix a chemical reaction occurs, that results in production of phenol and peroxyacid ester. Peroxyacid ester is later spontaneously decomposed to carbon dioxide (I bet you at least heard about this one), but this process results in release of energy that excites the dye (in chemical way of speaking). The dye really doesn’t like this excited state and relaxes by releasing a photon – thus light is being emitted. See the reaction bellow. What is great about this reaction is that it produces mostly light and almost no heat. The dynamics and light intensity are scalable to some extent – at low temperatures the glow stick lasts longer, but is dimmer, whereas increased temperature results in brighter light that wears of faster. Adjusting the concentrations of chemicals also leads to different dynamics. The dye is usually fluorescent (fluorophore) so when you are unsure what colour will the glowstick have just shine some UV light onto it. This is really quite practical with white and yellow ones as they look almost the same prior activation. Downside of glowsticks- they glow only once. But if you go to the UV party you can bring your already used glowsticks – they shine under UV light even after being used. Differently coloured sticks are created with use of different fluorophores. Either they mix more of them together or slightly different chemicals are being used.
I ate the glow stick. Will I die?
I firmly believe that none of you will ever eat a glow stick. Not only is it full of shards of glass, but it is full of phenol. What is phenol you ask? Well, not something good- it is a volatile and toxic chemical. If the light stick breaks and some of its contents get onto your skin then probably nothing happens. But. There are cases of redness, skin irritation or swelling, in extreme cases nausea and vomiting. And phenol is also very quickly absorbed through skin…so try not to spill too many glow sticks onto yourself throughout the party.

Conclusion
Well, I always keep at least one glow stick with me- in case I need light quickly or aliens happen to drop giant EMP bomb on us. Hopefully you did learn something new and if you enjoyed reading this article then support me with upvote, comment or break a glowstick for me.
Have a great day!
P.S. Here is a great video of glowstringing - art of using glowsticks to create this spectacular show!
References
Personal experience and Wikipedia article - Glow stick

I read now, but you will get the vote tomorrow, I need to recover :)
Ok, 75%, not bad...
Find me on chat/ Discord for that design I asked you (the same name)