Freezing and Boiling Point of Water: Misconceptions
Freezing and Boiling Point of Water: Misconceptions
Hi, its my second entry to Suesa's Science Challenge #2
So, you’re just sitting there on your desk thinking that you’ll become a millionaire as you stare at the screen while on your steemit account and you suddenly start realizing none of your posts are doing really well so you start pondering about your life and think that your life has always been a disappointment. You start throwing things around and the glass of water that was on your table spills over all over your pants. Now, you are sad and alone AND you pissed your pants. But you leave the pants alone for a day and it dries up (unlike your tears). What has happened? Did it just boil away? Yes it did! But how? Would you apply the same for your tears? Let’s find out.
What is the boiling point of water? Ask this question to few people around you. The answer would probably be 100 degrees Celsius. What if I told you that; water boils at 50 degrees Celsius or 150 degrees Celsius? You would probably freak out. But this is true. How? I will explain in this post. Stay tuned.
First, let me give you a simple definition of boiling point. The boiling point of water is the temperature at which the water changes into vapor. You may wonder why water changes to vapor. Water consists of H2O molecules bonded by intermolecular force called Vander Waal's force (AND HYDROGEN BONDING!). These are weak forces. When water is heated, molecules gain enough kinetic energy to break these bond and escape. If the pressure provided by escaped water molecules, called vapor pressure, is equal to atmospheric pressure, we say the temperature is at boiling point.
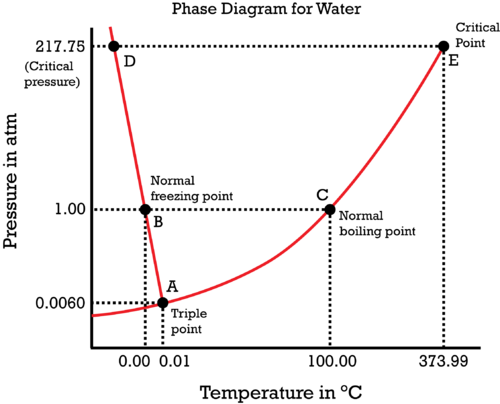
Now, what if the pressure above the liquid is reduced? Of course, the boiling pressure decreases as less heat is required to escape to form vapor. Hence, the boiling point of water depends upon more than just temperature. It also depends upon pressure. 100 degrees Celsius is the true boiling point of water at 1 atm pressure. At around 0.138 atm, the boiling point is 50 degrees Celsius and at about 4.75 atm, the boiling point of water is 150 degrees Celsius.
The same goes with freezing point, which also depends upon both pressure and temperature. The phase diagram of water shows the different states at particular temperatures and pressures.
Are you ready for another twist? If you aren't then spoiler alert!! That water boils at 100 degrees Celsius at 1 atm and freezes at 0 degrees Celsius is also not entirely correct. Was your entire life a lie? What is going on? Should you ask your parents? Yeah, if they are you real parents. This can be explained by of solidification processes.
Solidification is simply the process of formation of solid generally from liquid phase due to cooling. At first nucleation occurs. The solidification must start from some point. The formation of first nano crystallite from liquid is called nucleation. That is called a nuclei. Nucleation process is general process and hence occurs in all phase transformations (during gas-liquid transformation too).
Consider, the nuclei formed is of spherical shape and has radius 'r'.
The energy associated with the crystalline spherical nuclei is less than the energy associated with the liquid(water). This is the energy that liquid foregoes for solidification. Let, free energy per unit volume be ΔGv. It is negative as solidification is thermodynamically feasible.
During solidification, a solid-liquid interface is formed. It is a surface phenomenon. This amount of energy should be supplied to form stable nuclei. Let surface free energy per unit area be ΔGs. So, total free energy is given by;
For stable nuclei, Gibbs free energy should be negative. i.e ΔG < 0. ΔGv and ΔGs are not strong function of radius and hence can be assumed constant.
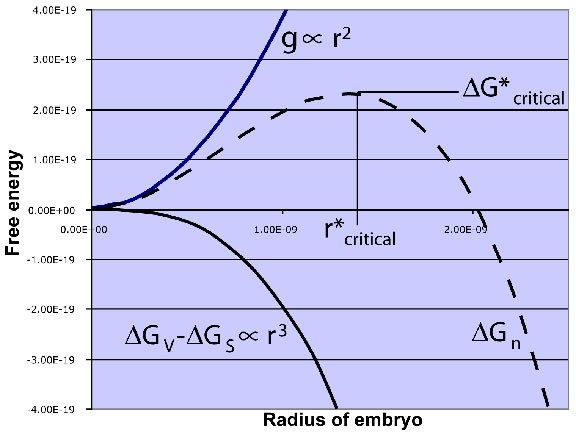
This radius is the critical radius(r*).
if the radius of nuclei greater than critical radius , the nucleus is stable and begins to grow.
if the radius of nuclei less than critical radius, the nucleus is unstable and do not grow.
At the thermodynamic equilibrium of solids and liquids i.e. at freezing temperature, ΔGv = 0, so the total ΔG will be positive. Hence, freezing does not start at thermodynamic freezing point. For the phase transition, the liquid temperature of should further decreased. Similarly, when liquid is to be converted to vapor, it should be super heated.
Conclusion
We can see that liquids do not really freeze at their freezing temperature and do not really boil at their boiling point! We need to under cool the liquid for it to solidify and super heat it for it to boil!
These misconceptions annoy me.
Sources:

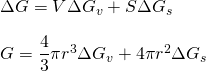
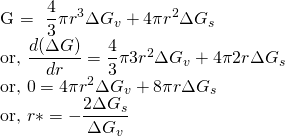
This post recieved an upvote from minnowpond. If you would like to recieve upvotes from minnowpond on all your posts, simply FOLLOW @minnowpond
This post recieved an upvote from minnowpond. If you would like to recieve upvotes from minnowpond on all your posts, simply FOLLOW @minnowpond
van der Waals! I guess you're more used to hearing it than reading it, which probably makes you a true scientist rather than a bookworm like me! :P
Good post, but it got suddenly technical at one point. I'm still not sure I understand why water boils off from clothes at room temp!
Boiling Water Until It Freezes (Watch this Video)
Can You (please) tell us something about the resonance frequencies of OH - , H +, OH_2 ? What will happen to water in different spots of the phase diagram when you apply these frequencies with different loudness/intensity ?