Haber-Bosch process. A form of Fertilize that Feeds the world.
AMMONIA
Anhydrous ammonia is a colorless gas, with an irritating and toxic odor. Because of its high latent heat of vaporization it is used as a refrigerant fluid, although it is dangerous in the event of leaks. It is very soluble in water, it hydrates forming NH4OH that ionizes , generating solutions of strong basic character.
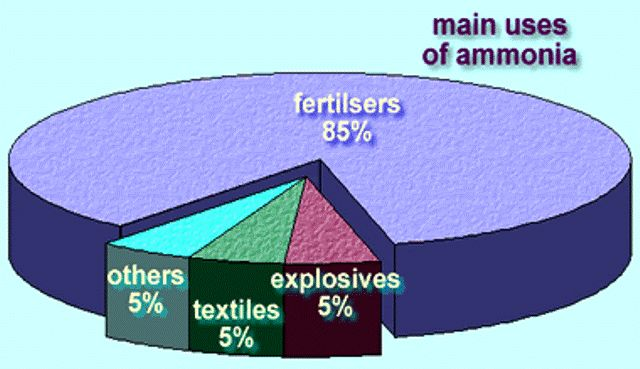
The ammonium ion is easily assimilable by plants. Due to its importance as a nutrient, it is the second most industrially produced chemical product on a world scale after sulfuric acid. It is the raw material for the manufacture of nitric acid, ammonium nitrate and other inorganic nitrates, as well as urea, all of them of massive use as fertilizers.
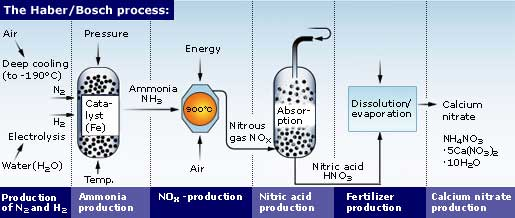
Ammonia production
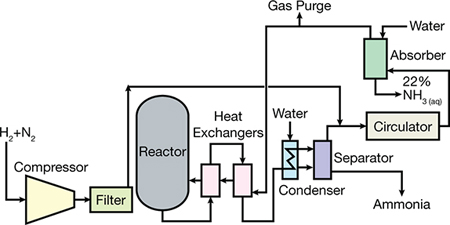
Its global production is based on natural gas and nitrogen in the air in plants. The natural gas is reformed first in an allothermic furnace and then in another autothermal furnace in which the air supplied by the nitrogen is introduced. Its predominant location is in proximity to oil wells, whose associated gas is a cheap raw material. In addition, liquid ammonia is stored and transported much more easily than natural gas and has a very wide and transparent market. Both cryogenic and semicryogenic tanks must have effective thermal insulation and emergency liquefaction systems. Ammonia is produced by a catalytic reaction of hydrogen and nitrogen at high temperature and pressure, the basic material being natural gas, from which hydrogen is obtained; the nitrogen for the process is obtained from atmospheric air. Ammonia is also produced, although in small quantities, as a by-product of the manufacture of coke. Ammonia is produced in nature by the action of putrefaction bacteria and the formation of ammonia on the organic matter of the soil. For this reason smells of ammonia are perceived in stables and pens, where this action takes place. Currently, around 80% of the ammonia produced worldwide is used as a nitrogen source to manufacture fertilizers, while the remaining 20% is used in various industrial applications, such as the production of plastics, fibers, explosives, hydrazine, amines, amides, nitriles and other organic nitrogen compounds that serve as intermediates in the manufacture of dyes and pharmaceuticals. Among the inorganic products that are made from ammonia are nitric acid, urea and sodium cyanide. Ammonia is also used in protective measures for the environment, for example, to remove NOx from combustion gases. Liquid ammonia is a prominent solvent and is also used as a refrigerant.
Fritz Haber and Carl Bosch
Nitrogen is an essential nutrient for plants, but they can not take it directly in the inert gaseous form present in the atmosphere; They need the microbes to do the work for them. Until the beginning of the twentieth century, only manure and nitrate from Chile, from the guano of birds, could supply nitrogen to plants in a usable form. This was so until July 3, 1909, the German chemist Fritz Haber (1868-1934) for the first time attaching nitrogen and hydrogen, at high pressure and temperature and through the use of a metallic catalyst, to produce ammonia. In the company BASF, Carl Bosch (1874-1940) was in charge of transforming Haber's experiment into an industrial-scale process. Both would receive the Nobel Prize in Chemistry, Haber in 1918 and Bosch in 1931.

The process of Haber-Bosch changed the world
it is estimated that the diet of half the world's population depends on the fertilizers derived from it. But it has a dark reverse; This method allowed the large-scale manufacture of modern explosives, responsible for between 100 and 150 million deaths in the last century. On the occasion of the First World War, Haber was also an enthusiastic promoter of chemical weapons, creating chlorine gas whose use in the trenches he supervised himself. It is believed that this Haber activity caused the suicide of his first wife, the chemist Clara Immerwahr, of pacifist convictions. The process consists in the direct reaction between nitrogen (coming from the atmosphere) and hydrogen (coming from natural gas), both in a gaseous state. It is an exothermic reaction so that an excessive temperature increase does not favor the formation of ammonia. However, the speed at which NH3 forms at room temperature is almost nil. It is a very slow reaction since it has a high activation energy, a consequence of the stability of N2. Haber's solution to the problem was to use a catalyst (iron oxide that is reduced to iron in the H2 atmosphere) and increase the pressure since this favors the formation of the product. Converting Haber's method into a manufacturing process was work done by Carl Bosh, the chemical engineer at BASF, who thus obtained his Nobel.