EXTRACTION, LIQUID-LIQUID. Essential process for the separation of chemical mixtures
liquid-liquid extraction
The liquid-liquid extraction is, together with the distillation, the most important basic operation in the separation of homogeneous liquid mixtures. It consists of separating one or more dissolved substances in a solvent by transferring them to another insoluble or partially insoluble solvent in the first one. The transfer of matter is achieved by direct contact between the two liquid phases. One of the phases is dispersed in the other to increase the interfacial surface and increase the flow of transferred matter. In a liquid-liquid extraction operation, the solution whose components are to be separated is referred to as the feed, the extraction solvent is used to separate the desired component, refined to the feed already treated and extract to the solution with the solute. recovered.
Ternary equilibrium diagrams.
In the design of a liquid-liquid extraction operation, it is usually considered that the refining and the extract are in equilibrium. The equilibrium data that must be handled will be at least those corresponding to a ternary system (two solvents and one solute), with two of the components immiscible or partially immiscible with each other.
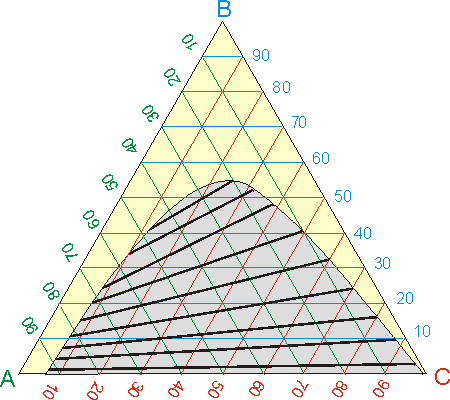
One of the most common ways of collecting equilibrium data in ternary systems is triangular diagrams. Figure 1 shows an equilateral triangular diagram. The vertices of the triangle represent pure compounds, a point on one side would correspond to a binary mixture and a point inside the triangle would represent a ternary mixture. The composition of a mixture can be determined by direct reading in the diagram, as shown in Figure 1. The concentration of the components in the diagram is shown as a mole fraction or mass fraction.
In systems of interest for liquid-liquid extraction, the two solvents involved are immiscible or partially immiscible with each other. That is, mixing them in the proper proportions can result in the formation of two phases. In addition, the presence of a solute modifies the solubility of one solvent in another. To represent this behavior, and be able to know if a given mixture corresponds to one or two phases, the liquid-liquid triangular diagrams have the so-called bimodal solubility curve (Figure 1). A mixture represented by a point located above the binodal curve will be constituted by a single phase. On the contrary, two phases correspond to a mixture located below the binodal curve.
The two equilibrium phases are linked by a distribution line. The distribution line passes through the mixing point and its ends on the binodal curve indicate the concentration of the two phases in equilibrium (Figure 1).
It is common to obtain reaction mixtures in aqueous solution or suspension (either because the reaction has been carried out in aqueous medium or because an aqueous solution has been added to the initial reaction mixture during the end of the reaction). In these situations, the extraction of the desired reaction product from this aqueous mixture can be achieved by adding a suitable organic solvent, more or less dense than water, which is immiscible with water and capable of solubilizing the maximum amount of product to extract but not the impurities that accompany it in the reaction mixture. After stirring the mixture of the two phases to increase the contact surface between them and allow a faster equilibrium of the product to be extracted between the two phases, a transfer of the desired product from the initial aqueous phase to the organic phase will take place, a greater quantity, the greater is its partition coefficient between the organic extraction solvent chosen and the water. A few minutes after the agitation, the two phases are separated again, spontaneously by decantation, due to the difference of densities between them, so that the organic phase containing the desired product can be separated by a simple decanting of the aqueous phase Containing impurities. The relative position of both phases depends on the density ratio. Since after this extraction, the aqueous phase frequently still contains a certain amount of the desired product, the extraction process is repeated a couple of times more with pure organic solvent. Once the extraction operation has been completed, the extracted product must be recovered from the combined organic phases. For this, the resulting organic phase must be dried with a drying agent, the resulting suspension filtered and finally the organic solvent removed from the dry solution containing the product extracted by distillation or evaporation.
GENERAL DESCRIPTION OF THE PROCESS
While distillation takes advantage of the different volatilities-that is, the different distributions of a product in the liquid and gas phases-liquid / liquid extraction is based on different solubilities - that is, the different distributions of a product in two. coexisting liquid phases. Therefore, for the extraction of a product (white spots) from the so-called feed liquor (blue liquid with white dots), it is necessary to find a suitable solvent (yellow liquid). The first step of an extraction process is the mixing in order to create an intense contact of the two liquid phases that allows the mass transfer of the product (white spots) from the liquor (blue) to the solvent (yellow). The second step is the separation of phases or the decantation of the 2 liquid phases. After the extraction of the product, the feeding liquor is called refined (blue liquid with fewer white spots), while the solvent containing the product is called an extract (yellow liquid with white spots). For the recovery of the product, the solvent must be separated from the product in a subsequent third step which is generally carried out by distillation.

SOLVENT REQUIREMENTS
The solvent should be checked with respect to the following characteristics:
- Maximum solubility of the product in the solvent
- Minimum solvent solubility in the refining
- Minimum solubility of the feed liquid in the solvent
- Rapid phase separation of the extract from refining
- Easy separation of the product from the extract/solvent
- With the suitable solvent, it is possible to carry out numerous applications conveniently by means of an extraction process, as described below.
Thanks for explaining about liquid extraction.
This reminds me of my organic & medicinal chemistry classes! Do you use this process in your day to day job?
Not exactly every day try to make an explanation of the process of chemical separation applied to the oil industry