TRANSITION ELEMENTS: Introducing Nitinol, a ‘shape memory’ alloy.
Nitinol is a remarkable alloy that can ‘remember’ shapes. Deform a piece of the alloy and it will return to its original shape after being heated. Nitinol is an alloy of nickel and titanium, both transition elements. It was discovered in the USA in 1962 quite by chance, when researchers in the defence industry were seeking ways to make titanium less brittle by adding nickel to it. They found that the temperature at which Nitinol returns to its original shape can be set anywhere between -100 °C and 100 °C by altering the amount of nickel in the alloy.

Nitinol wires. Petermaerki - Own work, CC BY-SA 3.0
Nitinol wire can be used to repair damaged or blocked arteries. The wire is wound round a tube of the same diameter as the inside of an artery. It is heated so that this coiled shape is remembered. The wire is then cooled and straightened. It is passed into the artery to be repaired. The temperature of the blood warms up the wire, which returns to its coiled shape. So an artery with a weak wall can be reinforced and a constriction in an artery can be unblocked. Nitinol does not react with body chemicals, and the arterial wall soon grows round it.
WHAT IS A TRANSITION ELEMENT?
Ask someone to name the first metal that comes to mind, and the chances are that a transition element will be named. It may be iron, essential to the construction of many buildings, most vehicles and almost all machinery. It may be gold, a precious metal used in jewelry and other forms of ornamentation, and as a protective coating. It may be copper, used in electric wiring and for indoor water pipes, or it may be silver or platinum, nickel or chromium.

A nitinol paperclip bent and recovered after being placed in hot water. Petermaerki, CC BY-SA 3.0
Everyone is familiar with the transition elements through their use, not in compounds, but on their own or in alloys, such as brass, bronze and steel.
The transition metal compound with the world’s largest annual production (4.5 million tonnes) is titanium(IV) oxide, TiO2. This compound is bright white when pure, and so is used as the pigment in white paint, white paper and white plastics, as well as in sunscreens.
Transition metals are essential to life. Cobalt is found in vitamin B1, where it acts as a catalyst. The iron in haemoglobin is involved in transporting oxygen around the body. And nickel and copper are essential components of several enzymes.
So what is a transition element? The central block of the Periodic Table is the d block. This article deals with the ten d block elements in Period 4, sometimes called the ‘first row transition elements’ as they are the top row of the d block. These elements have a closely similar set of properties, so it is tempting to call them all transition elements. However, on further examination, scandium at one end of the first row and zinc at the other are obviously different from the rest. This has led chemists to define transition elements as elements that form one or more ions with a partially filled d subshell. This definition excludes zinc and scandium.
ELECTRON CONFIGURATION
In the electron configurations of the first-row transition elements. You notice that the 4s subshell has already been filled because this is at a lower energy than the 3d subshell. The full electron configuration of titanium (atomic number 22) is 1s2 2s2 2p6 3s2 3p6 3d2 4s2.
Since the noble gas core is argon (1s2 2s2 2p6 3s2 3p6), titanium can be abbreviated to [Ar]3d24s2.
For each element from scandium to zinc, the number of protons increases by one. This increases the positive charge on the nucleus. However, because the electrons are added to the inner 3d subshell, the outer 4s electrons tend to be shielded from the increasing charge. This partly explains the similarity in physical and chemical properties. Also, because the 3d and 4s subshells have similar energies, the electrons from both can take part in bonding, which gives rise to some characteristic transition metal properties.
Electrons occupy first the orbitals of lowest energy. The orbitals in a subshell are first occupied singly by electrons that spin in the same direction, which helps to minimize the interelectron repulsion. Only when all the d orbitals in the d subshell are singly occupied do electrons start to pair up.
Electron configurations of chromium and copper
The chromium atom and the copper atom each have only one electron in the 4s subshell. As the number of protons increases, the number of electrons in the d subshell also increases by one each time, until chromium is reached. At chromium, there is a jump of two electrons, one of which is from the 4s subshell. Why is this? The half-filled subshell has five singly occupied orbitals. So, it is a lower energy arrangement to have 3d5 and only one 4s electron, as this removes the paired electron in the 4s orbital and thereby reduces repulsion between electrons. The more stable an arrangement, the lower its energy. At copper, the most stable arrangement is to have 3d104s1, since a full 3d subshell is a stable arrangement.
FORMATION OF TRANSITION METAL IONS
Metal ions are always formed by the loss of electrons, which requires energy. In the case of transition metal ions, the first electrons to be lost are in the 4s subshell. This is because, once the 3d subshell starts to fill, the 4s electrons are repelled further from the positive nucleus. This can be seen with Mn2+ as shown in the diagram drawn by me below.
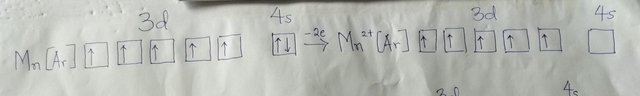
Thus, the electron configuration of Mn2+ is [Ar]3d5.
Fe loses its 4s electrons to form Fe2+ as shown in my figure drawn by me below.

Which has an electron configuration of [Ar]3d6.
As the energies of the 4s and 3d subshells are still fairly close together, 3d electrons may also be lost to form ions with charges of 3+. In forming Fe3+ a 3d electron is lost together with the two 4s electrons to give an electron configuration of [Ar]3d5 as shown below:
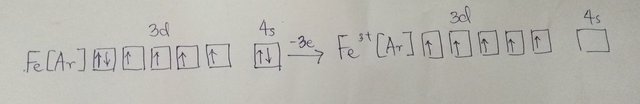
As already noted, scandium and zinc do not show typical transition metal properties. Zinc has an electron configuration [Ar]3d104s2. The stable full d subshell means that zinc forms only Zn2+ ions, [Ar]3d10. Recalling the definition of transition metals as those that form one or more ions with a partially filled d subshell, zinc does not qualify as a transition element.
HOW DO YOU RECOGNIZE TRANSITION ELEMENTS WHEN YOU SEE ONE?
Talking of physical appearance and properties, transition elements are typical metals. They are hard and dense, have high melting points and are good conductors of heat and electricity. They tend to be strong and durable, and have high tensile strengths, as well as other useful mechanical properties. So they find many applications, from bridges to cooking utensils. Contrast this with the s block metals, some of which are soft and have low melting points.
A more detailed examination of the physical properties reveals their close similarity. This is again explained by the electron configurations.
MELTING POINTS AND HARDNESS
The high melting points and the hardness of transition metals are caused by their strong metallic bonds. As is known that metallic bonding is caused by the delocalization of outer electrons, leaving positive metal ions surrounded by a sea of electrons. The more electrons that are delocalized, the stronger the metallic bond. In the case of transition metals, some 3d electrons can be delocalized along with the 4s. This explains why they have higher melting points compared with s block elements, such as potassium and calcium.
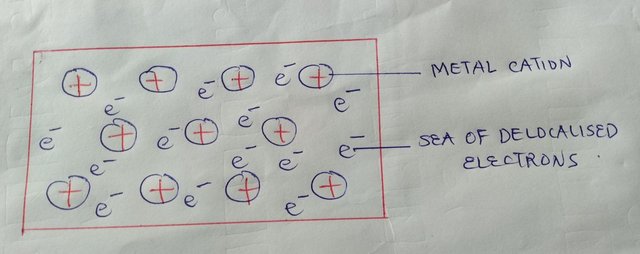
A figure drawn by me showing that metallic bonding in transition metals involves 3d as well as 4s electrons.
IONIZATION ENERGIES PROVIDE EVIDENCE FOR ELECTRONIC CONFIGURATION
Ionization energy has discussed in one of my article. Although it is a physical property, it is fundamental in determining the chemical properties of elements. The first ionization energy is the energy required to remove 1 mole of electrons from 1 mole of gaseous atoms to form 1 mole of gaseous ions: i.e
M(g) → M+(g) + e- ΔHi (1)
The second ionization energy is the energy required to remove the second mole of electrons, the first mole having already been removed:
M+(g) → M2+(g) + e- ΔHi (2)
So, in the first four successive ionization energies of the elements of the first transition series Sc To Zn. Ca and K are also included for Comparison. Note how similar all the first ionization energies are across this series. The same similarity is seen with the second ionization energies. This is because the 4s electrons are the first to be removed, even though it is the 3d subshell that is being filled along the series. This shows that the inner 3d electrons shield the 4s electrons from the increasing nuclear charge. In the case of the third and fourth ionization energies, the 3d electrons are being removed from the same subshell. So, the effective nuclear charge is increasing significantly along the period.

CONDUCTION OF ELECTRICITY
The conductivity of metals results from delocalization of the outer electrons in the metallic bond, which makes the electrons free to move under the influence of a potential difference. Transition metal atoms also have the 3d electrons as part of their metallic bond, which makes them better conductors than calcium, which can only use its 4s electrons. In fact, copper and silver are the best metallic conductors at room temperature.
WHAT ARE THE CHEMICAL PROPERTIES OF TRANSITION ELEMENTS?
There is a close similarity of the physical properties of the transition elements, So what about their chemical properties? There are four that characterize Transition elements: They are the variable oxidation states in their compounds, formation of complexes; formation of coloured ions; and catalysis of reactions both as elements and compounds. Let’s examine each one of them.
VARIABLE OXIDATION STATES
The terms oxidation state and oxidation number tend to be used interchangeably. However, when considering the transition elements, the term oxidation state is usually preferred.
Lower oxidation states in ionic compounds
There is a wide range of oxidation states in the transition metals. Contrast this with Group 1 and Group 2 elements, each of which has only one oxidation state (+1 and +2 for Groups 1 and 2, respectively). The difference is explained by the closeness of the 4s and 3d energy levels. In calcium, the two 4s electrons are easily removed, but to remove another electron means breaking into the 3p subshell which requires much more energy. This is not the case with the transition metals.
Remember that the first electrons to be lost in forming a transition metal ion are the 4s electrons, which makes the +2 oxidation state common, as in Fe2+ and Co2+. However, the 3d electrons may also be lost, which means that ions such as V3+, Fe3+ and Cr3+ are also common. The table below shows this.
Comparison of the first four ionization energies of calcium and iron
| Element | Electron configuration of element | ΔHi(1) | ΔHi(2) | ΔHi(3) | ΔHi(4) |
| Ca | 1s2 2s2 2p6 3s2 3p6 4s2 | 590 | 1145 | 4912 | 6474 |
| Fe | 1s2 2s2 2p6 3s2 3p6 3d6 4s2 | 759 | 1561 | 2958 | 5290 |
Notice the big jump in the third ionization energy
of calcium as the 3p electron is removed. In the case of iron, the increase is
more gradual and the simple ions Fe2+ and Fe3+ are both
formed. The electron configurations of Fe2+ and Fe3+ are:
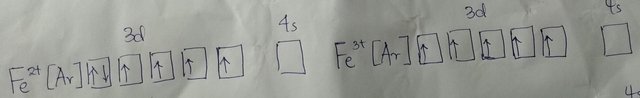
The electron configurations of manganese in the +2 and +3 oxidation states are:

In the figure showing all the common oxidation states of the first transition series. It is noticed that both scandium and zinc have only one oxidation state, which is yet another reason why they should not be classified as transition metals. From titanium to copper, both the +2 and +3 oxidation states exist. A first, the +3 oxidation state is common, which shows the greater stability of the M3+ ion. But from manganese onwards the +2 oxidation state becomes more common. The removal of a third electron from the d subshell is energetically more difficult after manganese, because the effective nuclear charge becomes so much greater than it is up to manganese. The reason for the relative stabilities of the +2 and +3 oxidation states is explored further when electrode potentials are considered (which is an explanation for another time).
Higher oxidation states
The maximum oxidation state increases across the first transition series, reaching +7 at manganese. Note that this corresponds to the total number of electrons in the 4s and 3d subshells. However, a Mn7+ ion or a Cr6+ ion would be too polarising to exist. Also, too much energy would be needed to remove so many electrons. The higher oxidation states are found covalently bonded either in simple compounds, such as TiO2, V2O5, CrO3 and Mn2O7 or as ions, such as VO2+, Cr2O72- and MnO4-. The availability of partially filled or empty orbitals, particularly in the 4s and 3d subshells, allows the higher oxidation states to be reached.
After manganese, the maximum oxidation state of each element decreases because of the increasing energy needed to involve 3d electrons in bonding, and because singly filled orbitals are required in order to form a covalent bond.
SUMMARY
Nitinol is a very important alloy which is gotten from the combination of two transition elements; Nickel and titanium. It’s majorly used in medicine for repairing damaged arteries.
Basically, transition elements are those elements that form one or more ions with partially filled d block. They are very useful with a lot of applications. They are verily used in construction, electric wiring, machineries and also as jewelleries.
The most widely produced transition element is Titanium (IV) oxide, TiO2 which is very useful in paint and sunscreens.
In terms of electron configuration, transition elements are those that have their 4s subshell filled first before the 3d subshell. They also form transition metal ions. Furthermore, transition elements are easily recognized from their appearance and properties; they are hard with high melting points because of their strong metallic bonds and very good conductors of electricity.
In regards to their chemical properties, transition elements do have variable oxidation states, some are low while some are very high like those of Mn2O7 which is having an oxidation state of +7.
REFERENCES
https://www.technology.matthey.com/pdf/66-76-jmtr-jan17.pdf
https://en.wikipedia.org/wiki/Shape-memory_alloy
https://www.imagesco.com/articles/nitinol/01.html
https://en.wikipedia.org/wiki/Transition_metal
https://www.britannica.com/science/transition-metal
https://www.brightstorm.com/science/chemistry/the-atom/exceptions-to-electron-configuration/
https://courses.lumenlearning.com/cheminter/chapter/transition-metal-ion-formation/
https://www.ausetute.com.au/trmetals.html
https://www.chemguide.co.uk/atoms/properties/3d4sproblem.html
https://www.jstor.org/stable/96578
https://arxiv.org/pdf/1102.5654
https://royalsocietypublishing.org/doi/abs/10.1098/rspa.1936.0031
https://www.thoughtco.com/transition-metals-606664
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app and included @steemstem in the list of beneficiaries of this post. This granted you a stronger support from SteemSTEM.
Congratulations @empressteemah! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
You can view your badges on your Steem Board and compare to others on the Steem Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPVote for @Steemitboard as a witness to get one more award and increased upvotes!