Molecules in Interstellar Space and a Close Look at Electrons
MOLECULES IN INTERSTELLAR SPACE
The giant gas clouds in interstellar space –
the space between stars – contain vast amounts of very thinly dispersed atoms,
ions and molecules. We know what this material consists of because each type of
atom, ion or molecule absorbs and emits energy of particular wavelengths across
the electromagnetic spectrum, depending on the arrangement of its electrons.
From Earth, we can detect and record the patterns of these absorbed and emitted
wavelengths: each type of particle has its own pattern, which reveals its
presence, rather like a fingerprint. Hydrogen, for example, emits radio waves
at a wavelength of 21 cm.
Chemists are very interested in the molecules
of interstellar space. So far, they have identified over 200. Some are also common
on Earth, such as hydrogen chloride, carbon monoxide, water and ethanol. Others
are unusual, and several were discovered in space before they were even
identified in the laboratory. An example is a three-member carbon ring, C3H2,
an interstellar molecule widespread in our Galaxy and common in others, but too
unstable to be made on Earth.<o:p></o:p>
So how do these molecules form? The
temperatures and pressures of interstellar space are just too low for atoms to
meet, collide and join together as they do on Earth. However dust particles in
space provide a surface on which reactions are catalyzed. Also gases could
condense on the grains, be bombarded by cosmic radiation and form more complex
molecules. Some scientists think that these dust particles may have fallen onto
young planets, seeding them with the chemicals for life. There is recent
evidence that glycine (NH2CH2COOH), an amino acid that is
one of the building blocks of our proteins, exists in interstellar space.<o:p></o:p>
INTRODUCTION: Electrons.<o:p></o:p>
The glorious colours of fireworks, the
discovery of helium in the Sun before it was discovered on Earth, our knowledge
of the atoms and molecules in distant stars – all these are connected with electrons. To a chemist, the most
interesting part of an atom is its electrons, because it is the electrons, not
the protons or neutrons, that account for every chemical reaction.<o:p></o:p>

We cannot observe electrons directly, but in
this chapter you will find out how our knowledge about them has grown and
developed. The fact that we can know things without direct observations is a
key point in understanding the nature of scientific knowledge.<o:p></o:p>
Scientists in the 19th century investigated the
light that materials absorb and emit, and went on to discover new elements. In
the 20th century, our understanding of the nature of light led scientists to
release the energy of lasers.<o:p></o:p>
ALL THE COLOURS OF THE RAINBOW<o:p></o:p>
In 1666, Sir Isaac Newton first recorded the
fact that, when visible light was passed through a prism, it was split to
produce a continuous spectrum of rainbow colours that
contained all the wavelengths of light.<o:p></o:p>
Robert Bunsen is best known for inventing the
Bunsen burner. But, with Wilhelm Kirchhoff. He invented an even more important
instrument for the progress of chemistry – the spectroscope. When put in a
flame, compounds that contain sodium were known to colour the flame yellow;
potassium compounds coloured it lilac. The spectroscope took these observations
and Newton’s visible spectrum a step further.<o:p></o:p>
In the spectroscope, light passes through a
narrow slit and a prism, and forms a spectrum. When Bunsen and Kirchhoff viewed
the sodium flame with this light, they saw bright lines (images of the slit) in
the yellow part of the spectrum and some less bright lines in other parts. They
viewed light from compounds of other metals in the same way, and soon realized
that each element had its own characteristic fingerprint of lines in different
parts of the spectrum.<o:p></o:p>
With this technique, they discovered the elements
rubidium and caesium while examining the spectrum of a lithium ore. They found
strong red and blue lines which could not be accounted for by the elements
known to be in the ore. Rubidium is taken from the Latin for ‘red’ and caesium
is named after the Latin for ‘blue’.<o:p></o:p>

Rubidium spectrum; 400 nm - 700 nm,
McZusatz (talk) - Own work, CC0.
As elements were being discovered and
identified by their spectral fingerprints ,astronomers were soon pointing
spectroscopes at the stars to look for characteristic lines of elements and
finding that the cosmos was made up of the same elements as the Earth.<o:p></o:p>
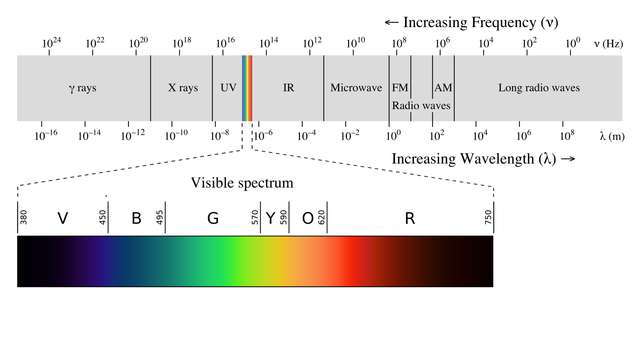
THE ELECTROMAGNETIC SPECTRUM<o:p></o:p>
The light our eyes detect is just a small part
of a very much wider electromagnetic
spectrum. This spectrum is made up
of all the types of electromagnetic radiation, and includes X-rays used by
dentists, the microwave radiation used to heat food in microwave ovens and
radio waves that bring us radio and television signals. The figure below shows
the continuous spectrum of electromagnetic radiation, called continuous because
all the wavelengths are represented. We shall now look more closely at electromagnetic
radiation to help us understand electrons and how they are arranged in atoms.<o:p></o:p>
As its name suggests, electromagnetic
radiation is made up of two components: an electrical and a magnetic one.
Electromagnetic radiation is one of the ways energy is transmitted through
space. It is why we feel the warmth of the Sun on earth, and when we suffer
sunburn if we have too much ultraviolet radiation.
 <o:p>
<o:p>
</o:p>
Electromagnetic spectrum, Philip Ronan, Gringer, CC BY-SA 3.0<o:p>
</o:p>
LIGHT AS WAVES<o:p></o:p>
Electromagnetic radiation is usually thought
of as waves and, like any wave, it can be described by its wavelength, frequency
and speed. Wavelength is measured in
metres (m), and its symbol is the Greek letter λ (lambda). Frequency, f, is measured in hertz (Hz), which means cycles
per second, and which can also be written as s-1. The speed of
electromagnetic waves is the speed of light, with the symbol c,
which is measured in metres per second (m/s). The terms are related in a simple
equation:<o:p></o:p>
c = λ ×
f<o:p></o:p>
(ms-1) = (m)
× (s-1) <o:p></o:p>
EXAMPLE<o:p></o:p>
Q: The average wavelength of a red traffic light is 750 nm. What is the
frequency of this radiation?<o:p></o:p>
A: First, you need to convert the average wavelength to metres:<o:p></o:p>
750 nm is 750 × 10-9 m, and the speed of light c is 3.00 × 108 ms-1.
<o:p></o:p>
Inserting these values into the equation:<o:p></o:p>
3.00 × 108
= λ × 750 × 10-9<o:p></o:p>
Rearranging: λ = 3.00 × 108 / 750 × 10-9 = 4 × 1014<o:p></o:p>
So the frequency of the red light is 4 ×
1014 Hz.<o:p></o:p>
LIGHT AS PARTICLES<o:p></o:p>
Up to the beginning of the 20th century, scientists
were agreed on a model of electromagnetic energy in which it was radiated and
absorbed by matter in the form of waves. The model did not explain all the
observations. Notably why heated elements emitted radiation as discontinuous
spectra with separate lines.<o:p></o:p>
In 1900, Max Planck proposed that particles
such as atoms and molecules absorb and emit energy in discrete (separate)
amounts or packets called ‘quanta’<o:p></o:p>
Planck’s equation is:<o:p></o:p>
E = hf<o:p></o:p>
Where E
is the energy in joules (J). h is Planck’s
constant, with a value of 6.626 × 10-34
Js, and f is the frequency in hertz
(Hz or s-1).<o:p></o:p>
A quantum
of energy is a precise packet of energy. These packets can have different
energy values, depending on their source, but you cannot have parts of packets,
only whole ones.<o:p></o:p>
THE PHOTOELECTRIC EFFECT<o:p></o:p>
In 1905, Albert Einstein used Max Planck’s
equation to explain another phenomenon that baffled scientists, the photoelectric effect. This is the release of electrons by some metals when light
is shone on them.
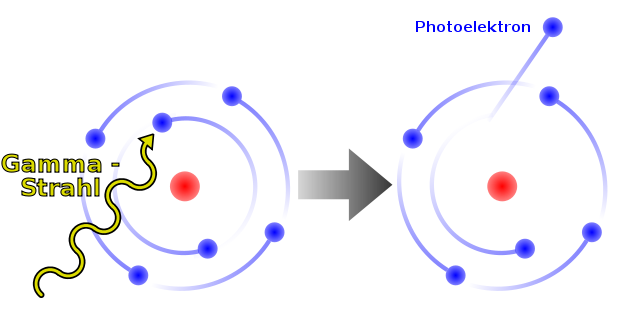
Illustration of the Photoelectric Effect, Dirk Hünniger - Own work, CC BY-SA 3.0.
It was found that the light had to be above a minimum
frequency (and so a minimum energy) before electrons were emitted. It did not
matter how intense (bright) the electromagnetic radiation was below this threshold frequency: only with a high
enough frequency were electrons released. Then, the more intense the light, the
greater the number of electrons released. According to the old wave-only model,
the electrons would absorb even low-energy radiation and eventually release it.
Einstein developed Planck’s idea of quanta, saying that some of the properties
of electromagnetic radiation could be explained only if light were thought of
as consisting of particles, which we now call photons. He also said that each photon is associated with a
particular quantum (amount) of energy, linked to its frequency by Planck’s
equation: E = hf.<o:p></o:p>
The photoelectric effect could now be explained.
Negative electrons are held in the atom by electrostatic forces of attraction
to the positive protons in the nucleus. When a photon collides with an
electron, the photon gives up its energy to that electron. If the energy is high
enough, the electron breaks free of the atom. The more intense the radiation,
the greater the number of photons, so more electrons are released from
materials that the radiation reaches. In digital cameras, for instance, photons
enter the lens and strike a grid of pixels, where they release electrons due to
the photoelectric effect. The charge from the electrons provides the digital
values for each pixel, and depends on the amount of light.<o:p></o:p>
THE WAVE-PARTICLE NATURE OF LIGHT<o:p></o:p>
In 1905, Einstein wrote three papers. One was
on his famous theory of special relativity, another an explanation of Brownian
motion. His third, an explanation of the photoelectric effect, gained him a
Nobel Prize in 1921, three years after his great friend Max Planck received
one. All three papers had a profound effect On 20th-century science and
Einstein was seen by many as one of the two greatest scientists that ever
lived, the other being Isaac Newton.<o:p></o:p>
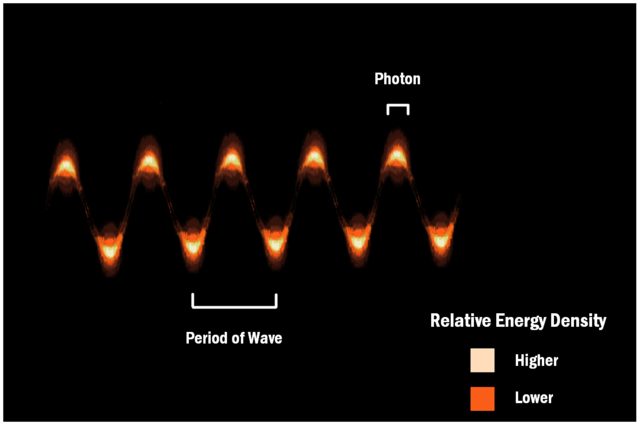
I have noted some observations suggesting that
light is made up of particles and others suggesting it is made up of waves. So
we now think of light as having a dual
nature, of both particles and waves. This wave-particle
duality is not just confined to
light; matter, too, can sometimes behave as if it were made up of waves.
 <o:p>
<o:p>
</o:p>
Wave-particle duality representation of a light wave, ThreePhaseAC - Own work, CC BY 4.0<o:p>
</o:p>
A big thanks to @emperorhassy, for helping me to co-author the aspect of the electromagnetic spectrum and light as a wave. In my next article, I’ll still be discussing
more on electrons touching the aspects of atomic emission spectrum of hydrogen,
Niel Bohr’s model of the hydrogen atom and a modern model of how electrons are
arranged amongst other nice topics.<o:p></o:p>
Thanks for coming.<o:p></o:p>
REFERENCES
List of interstellar and circumstellar molecules
Complex organic molecule found in interstellar space - BBC News
Molecules in interstellar space | Science
Unprecedented look at electron brings us closer to understanding the universe
Extremely close look at electron advances frontiers in ... - EurekAlert!
electromagnetic spectrum | Definition, Diagram, & Uses | Britannica.com
Electromagnetic Spectrum - Introduction - Imagine the Universe! - NASA
Electromagnetic spectrum - Wikipedia
You are not going to get all wavelengths of light. A prism refracts light. Microwaves are not going to appear when you refract light from the sun using a prism :P
Hello @mathowl, thanks for coming and also for the comment, I do appreciate your time.
But as regards your comment:
It is when you only pass visible light through a prism you get a continuous spectrum of rainbow colours which have all the wavelengths of light.
Mind the term "visible light". Of course, microwave is not the same as visible light and a prism only get to refract visible light not microwave.
Visible light waves consist of different wavelengths. The colour of visible light depends on its wavelength.
source
I'll be looking forward to many more of your comments.
Thanks.
Yes, that is correct. Thank you for your editing your post accordingly :P
Posted using Partiko Android
U're always welcome, @mathowl.
This remembers me two things I wrote on Steem two years ago: this old post of mine, as well as the following one.
You may want to check the follow up of this quantum mechanics series that could actually be useful (who knows?). :)
Congratulations @empressteemah! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
You can view your badges on your Steem Board and compare to others on the Steem Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPTo support your work, I also upvoted your post!
Vote for @Steemitboard as a witness to get one more award and increased upvotes!
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie.
If you appreciate the work we are doing, then consider supporting our witness stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having added @steemstem as a beneficiary to your post. This granted you a stronger support from SteemSTEM.
Thanks for having used the steemstem.io app. You got a stronger support!