Pretty blue crystals of cupric sulfate
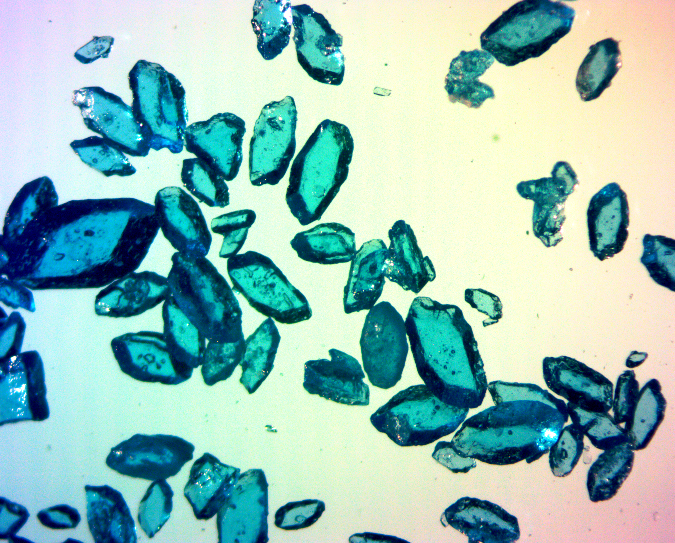
Given how well my picture of red cobalt(II) chloride went over for @mcw's #sciencepic contest, I thought I might do another post. This time, it features cupric sulfate (CuSO4·5H2O), which is in my opinion the most pleasantly colored inorganic compound generally found at a lab bench. Beyond aesthetics, the shade of blue is nice because it's a welcome relief from finding some white 'mystery powder' next to the analytical balance. Show any chemist a pile of dust this shade of blue, and unless you're intentionally trying to fool them, they'll likely guess what it is.
Like the cobalt in cobalt chloride, the copper in cupric sulfate is in the 2+ oxidation state and the color of its crystals depends on the water of hydration. The pentahydrate here is the one known for its blue color, with no water of hydration the anhydrous form is a boring white powder.
The crystals here were imaged on a dissecting microscope a low magnification with both transmitted and oblique lighting. I adjusted the white balance for the background, but did nothing particularly special to make the blue color stand out.
Cupric sulfate has been known to civilization since at least ancient Rome, where it was produced while leaching copper ores and its 'natural' mineral form is known as chalcanthite. An interesting side-note is that it is also known as roman vitriol, with vitriol indicating that it is associated with sulfates. This is interesting (at least to me), because my favorite 'archaic' chemical term is 'oil of vitriol' for sulfuric acid.

An example of chalcanthite. Source
It has a few uses in the lab, I commonly use it as a source of micronutrients for growth media and occasionally use it as part of an assay to test for certain kinds of sugars. I generally don't use it as a reagent in chemical synthesis, and, although it can be used to test for proteins, we use other assays for that.
It's also got a ton of niche industrial uses, including acting as a blue pigment for concrete, but the one most people outside of the lab probably know it for is as an herbicide and fungicide. I distinctly remember as a child watching people use it in their toilets to discourage tree roots from growing into the sewer lines (don't do this!). It's still used today as a component of burgundy mixture, which is used to protect grapes from fungal rot. So the next time you drink a glass of wine, think of roman vitriol.
Cheers!

Source