MAIN MAGNETIC BEHAVIORS
Hello Community Steemit. In this section we will discuss a little about the different types of magnetisms. By appropriate methods, the magnetic properties of any substance can be measured and classified as diamagnetic, paramagnetic, ferromagnetic, etc.
Diamagnetism
All the materials show some degrees of diamagnetism, which is characterized by a weak and negative magnetic susceptibility. The prefix DIA means "Against" or "through". For a diamagnetic substance, a magnetic field induces a magnetic moment opposite to the applied magnetic field that produced it. It is a property that makes a small contribution to the response of the material to the magnetic field, however, for materials that show some other forms of magnetism, the diamagnetic contribution becomes negligible.
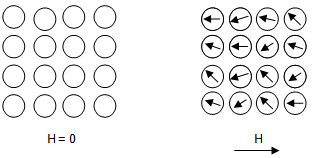
Paramagnetism
The paramagnetism corresponds to a positive susceptibility such that an applied magnetic field induces a magnetization which is aligned in parallel with the applied magnetic field that generates it. It is a behavior that is characterized by the existence of a net moment in the presence of an external magnetic field, but the interaction between the spins is very weak, and consequently its orientation is totally independent. However, when the temperature decreases, the thermal agitation decreases, favoring a progressive short-range ordering, which results in an increase in the magnetization when the temperature decreases.
The electronic paramagnetism appears in:
• Atoms, molecules and network defects that have an odd number of electrons since, in this case, the total spin of the system can not be zero. Examples: sodium-free atoms, gaseous nitric oxide (NO), free organic radicals, such as triphenylmethyl C (C6H5) 3, center F, in alkali halides.
• Free atoms and ions that contain an incomplete layer, transition elements, ions that have the same electronic structure as transition elements, elements of rare earths and actinides. Examples: Mn2 +, Gd3 +, U4 +. Many of these ions, although not always, are paramagnetic when they form a solid.
• Some compounds that contain an even number of electrons, such as molecular oxygen and some double organic radicals
• Metals.
Ferromagnetism
A ferromagnet has a spontaneous magnetic moment even in the absence of an applied fiel. This suggests that the electronic spins and the magnetic moment are organized in a regular way. It is important to point out that above the temperature of Curie Tc a ferromagnet becomes a paramagnet and its susceptibility will then follow the Curie-Weiss law. This fact led Weiss to assume brilliantly that a molecular field acts on a Ferromagnetic substance below its Curie temperature as well as above, and that this field is so strong that it can magnetize the substance to saturation even in absence of an applied field.
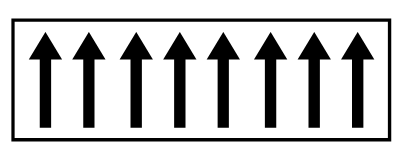
Ferrimagnetism
The ferrimagnetic substances show a substantial spontaneous magnetization at room temperature, as ferromagnetic, this fact alone makes them important industrially. Like the ferromagnetic, they consist of magnetically saturated domains, and show the phenomenon of magnetic saturation and hysteresis (tendency of a material to retain one of its properties, in the absence of the stimulus that has generated it).
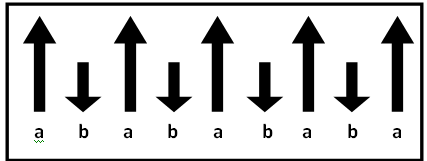
Ordering spins in Ferrimagnetic materials.

[1] B.D. Cullity, “Introduction to magnetic materials”. Addison- Wesley Publishing Company. London – Amsterdam 1972.
[2] S. Blundell, “Magnetism in condensed matter”. Oxford master serles in condensed matter physics. University of Oxford (2003).
[3] C. Kittel, “Introduction to solid state physics”. John Willey & sons, INC. 8th edition, New York, April. (2005).
nice work. upvoted and followed.
this is my blog(please upvote and follow if you like):
https://steemit.com/life/@benainouna/10-reasons-why-plastic-bags-should-be-banned
Thank you. I follow it