THE FUTURE OF GREEN ENERGY SOURCE - FUEL CELLS - IMPROVED BY RESEARCH
ImageSource
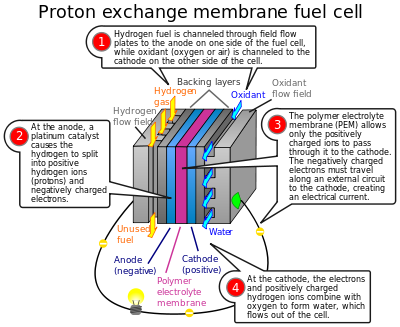
Fuel cells generate energy from fuels without combustion which releases harmful by-products. It does this through a chemical reaction that is more like reverse-electrolysis releasing a harmless by-product to the environment. This is what makes it eco-friendly and encouraged.
A single fuel cell should contain two electrodes - anode and cathode,an electrolyte, a source of fuel and a source of oxygen.
But how does this work? Why has this not been completely adopted? Is there a possibility that this will be adopted soon?
Chillax!
Your Questions are about to be answered.
😁😉😁
HOW FUEL CELLS WORK.
Fuel cells precisely generates electrical energy from fuels through a somewhat reverse-electrolytic process.
The electrolyte must be one which allows appropriate electrically charged particles through. If it allowed charged particles indiscriminately, it would disrupt the chemical reaction. There is a wide range(from liquids to solids) of electrolytes like these e.g alkali, molten carbonates, phosphoric acids, proton exchange membrane(PEM), solid oxides e.t.c
Often times the name of the electrolyte, gives the name of the cell.
Consider a hydrogen fuel source fuel cell. Fuel cells cells create current electricity through oxidizing the fuel at the anode, by stripping off ions from the fuel at the anode. While the electrons go out from the cell through a wire to power a load(electric motor, lightbulb), the oxidized fuel, in this case H+, flows through the electrolyte to the cathode. At the cathode, Oxygen picks up the electrons from the electrical circuit and then combines with the hydrogen ions from the anode via the electrolyte, forming water which is drained out of the cell. This creates a complete circuit for the electrons making current flow possible. In some other cells, Oxgen picks up electrons, travel through the electrolyte and then meet up with the hydrogen ions at the cathode. The water formed through this electricity-generation process is pure and drinkable.
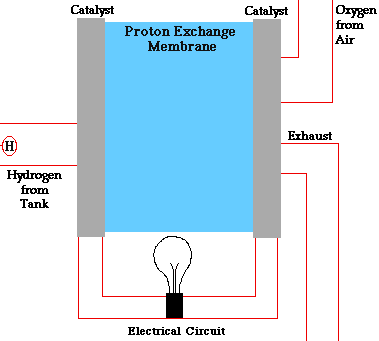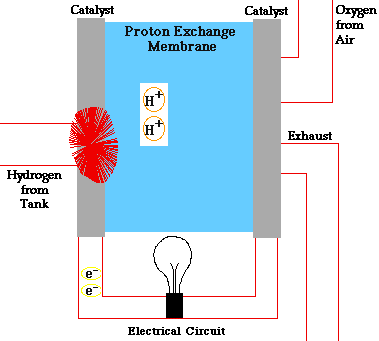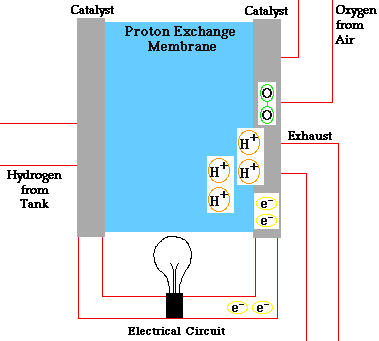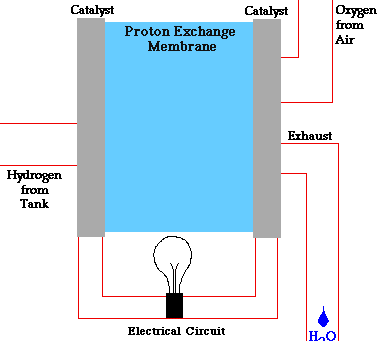
GifSource
The inventor of the fuel cell, Sir William Robert Grove also known as "The Father of Fuel Cell" was a Welsh Physicist and Barrister conceived this idea in 1839.
Fuel cells are not bound by thermodynamic laws - The carnot Limit, as they do not create energy through combustion. Hence they have higher efficiency and produces cleaner energy.
PROBLEMS INHIBITING ITS ECONOMIC AVAILABILITY.
Although the theoretical workings of a fuel cell are easy to illustrate, building one is complicated and can be difficult. Scientists, engineers and fuel cell developers are faced with the problem of the appropriate electrolytes to be used, design of efficient and long lasting electrodes which blend together with a chosen electrolytes. This has not completely stopped the adoption of fuel cells. NASA powered the Apollo space craft with an alkali fuel cell, providing both fuel and water!
IMPROVEMENT BY TODAY RESEARCH.
Today, solid oxides fuel cells have gained the attention of scientists because of its ability to directly convert fuels such as biomass, LNG and LPG to electrical energy.
The cathode of most fuel cells today have the perovskite structure(ABO3). A perovskite is a substance that has the same crystal structure as calcium titanium oxide (CaTiO3), named so because of its russian mineralogist founder L. A. Perovski(1792 - 1856).
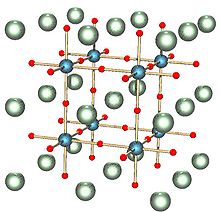

The performance of solid oxide fuel cells are to a large extent determined by the cathode, where reduction reaction of oxygen occurs. Although at initial operation, the performance of the perovskite structured cathode is high, this peformance decreases with time, limiting their lifespan of use.
From Computational and experimental research, it has been found out that the local compressive states of the strontium atoms in the perovskite lattice structure of the electrode, weakens Sr-O bonds which in turn promotes Strontium segregation. The strontium segregation phenomenon is a surface phenomenon where second phases like Strontium oxides (SrOx) accumulates on the surfaces of the solid oxides and electrodes, which results to a decrease in the performance of the electrodes.
The research team of the Korea Advanced Institute of Science and Technology (KAIST) led by professor Woochul Jong, reported to have observed strain changes in the perovskite oxide lattice structure which was responsible for this strontium segregation. By doping the cathode material with different sizes of metals, the lattice strain which led to the segregation was effectively inhibited.
In the words of professor Woochul Jong,
"This technology can be implemented by adding a small amount of metal atoms during material synthesis, without any additional process. I hope this technology will be useful in developing high-durable perovskite oxide electrode in the future."
Long lasting electrodes and Solid Oxide electrolytes which convert a long range of fuel to electrical energy is just the right recipe for a commercially available fuel cell.
An eco-friendly fuel use system is close by.
SOURCES
- http://americanhistory.si.edu/fuelcells/basics.htm
- https://www.sciencedaily.com/releases/2018/01/180118100814.htm
THANKS FOR READING;
CED.
Congratulations @ced000! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOPNice informative post...well done
Well done sir!
Thanks for stopping by
Amazing
informative as very educative
Thanks for sharing @CED000
Thanks sir