Experimental determination of the electron charge based on the Millikan experiment
During the period from 1909 to 1913, Robert Millikam developed a brilliant set of experiments in which he measured the charge of the electron, and demonstrated the quantized nature of this charge. the apparatus used includes 2 parallel metal plates, an atomizer with oil whose charged drops pass through a small hole in the upper plate, a beam of light directed horizontally to illuminate the drops of oil, which are observed by a telescope whose axis is in the angle with the beam of light. When the drops are seen in this way, they appear as bright stars against a dark background, where the speed of falling of individual drops is determined [1].

credits
An individual drop of mass m, charged, rising or falling between the plates, has four associated forces which are: the force of gravity, the drag force in a viscous medium, the force exerted by an applied electric field and the force of floating of the air that displaces the drop. Then you have that the force of gravity is always down, the drag force can go up or down since it is always opposed to the movement and will depend on whether the drop of oil goes up or down, the force exerted by the electric field depends on the experimenter, in this case applied in a way that exerts a rising force for the drop, which is why in the developed research the electric force is equally ascending and finally the floating force that is always rising [2].
These forces are expressed from (1) to (4) below:
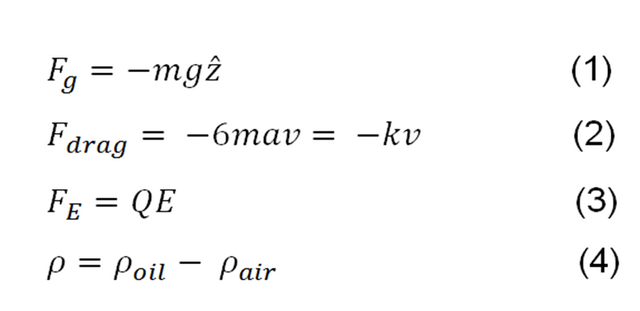
In Fg g is gravity and z is a unitary vestor in the direction of +z, the drag force comes from Stokes' law which describes the force of friction on a spherical body that moves in a viscous fluid (3), where n is the viscosity of the air, k is a positive constant included at convenience and v is the velocity of the drop when going up or down, the sign - indicates that this force always opposes the movement, the electric force depends on the potential difference between the plates, and finally the floating force that is decided to name it as a subtraction of the densities of the fluids used [2]. By the action of these 4 forces acquired by the drops after a certain time a terminal velocity is obtained so that the radius of the drop is expressed by [4]:

And from this same expression can derive the rate of descent of the drops, however, in this research work both speeds are calculated from the basic expression v = x/t.
Using the basic rule of classical mechanics "the sum of all the forces applied on a body must be equal to zero, only if it reaches the constant terminal velocity", then we have to:
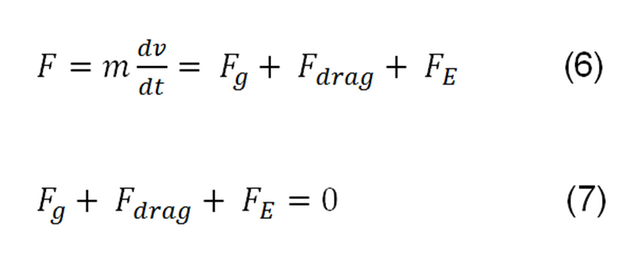
Starting from here and applying some mathematical relationships, an expression is obtained to determine the charge of each drop, the equation is given by:
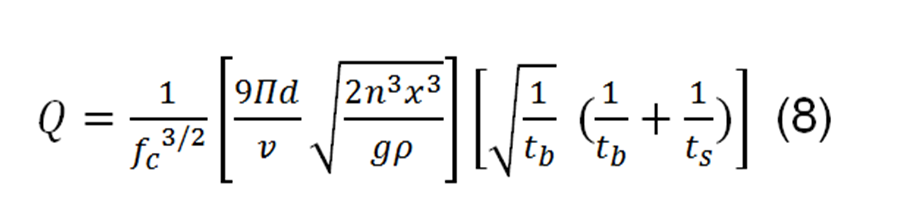
Where Q, is the total charge of the drop.
fc, is a correction factor for environmental conditions.
d, is the distance between the plates.
V, is the applied voltage.
And the other variables were named above. The correction factor can be written as:

And rc is given by:

Therefore, when carrying out a "high" number of time measurements that take the drops ascending and descending the same distance d, activating and deactivating the electric field, it is possible to calculate the charge of the drops from the Millikam experiment, which will be obtained as a result a quantization of the electric charge.
A type of montage for the Millikam experiment is shown below (unfortunately I do not have an original photo of the experiment that I made, since it was about 4 years ago and I lost the photos of the montage):

Real apparatus used for the experiment.
credits
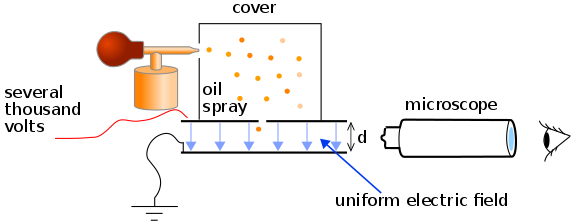 )
)Scheme of the Millikan experiment to determine the electron charge.
credits wiki
After making measurements on thousands of drops, Millikan and his collaborators found that all the drops had a charge equal to a whole multiple of the elementary charge with an accuracy of 1% [1].
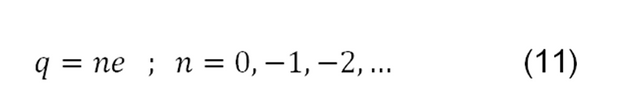
Where e = 1.60E-19 C, for this work was honored with the Nobel Prize in Physics in 1923.
It is necessary to calibrate the instrument first, matching a pair of parallel lines that contains the telescope with some printed on a plate so that the distance between the plates is effectively 2.99 mm and orient the focus of the telescope with the opening of the plates that generate an electric field, and where charged droplets will be found. Then the piece containing the plates is mounted, this is adjusted with the telescope's vision and a level is used to verify that it is properly adjusted, that is, correctly calibrated. Once all the above is done, the respective connections with the power source will be made, and the measurements will begin.
An atomizer is used to supply drops of oil charged to the system, which will then be accelerated upwards and downwards, in the first case thanks to the help of an electric field, and in the second case by action of the gravitational field.
In this work we took a data of 500 time values, both of rise due to the effect of the electric field, and of descent by action of gravity. From these times with the expressions previously mentioned, it was calculated: the radius, the speed of rise and fall of the drops, the correction factor and finally the drop load.
By statistics, it is known that the larger the population, the smaller the error and the discrepancy in measurement will be. Also, with a measurement table with a large data, those data that are far from the average can be disregarded.
The table below shows some of the values closest to the constant we are looking for, for this data collection procedure, 25 values of 500 measurements were taken and a discrepancy of 15.04% was obtained.
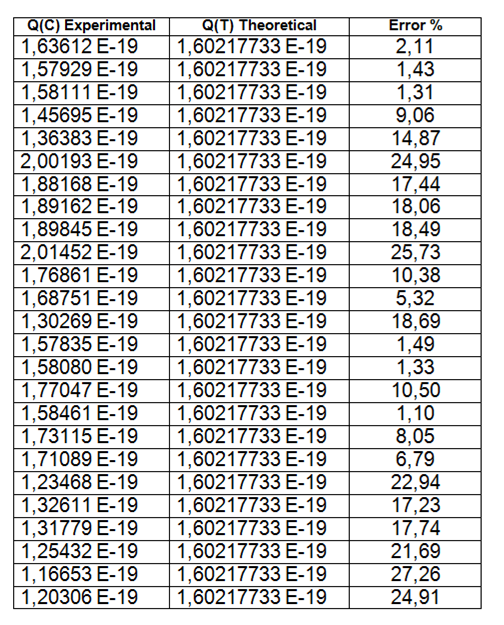
Table: Data obtained and its theoretical discrepancy.
In this case, a probable value of the electron charge was obtained from:
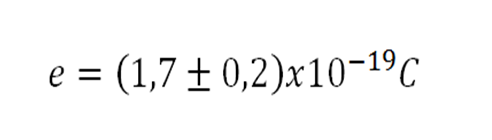
With a discrepancy of 15.04%.
Through the experiment carried out by Millikan in the 1900s it was possible to reproduce the experiment in the advanced laboratory of Physics, of the Experimental Faculty of Sciences at the University of Zulia. The experiment was carried out correctly taking into account different environmental and maneuvering factors that may affect the measurements.
the use of the expressions described from (1) to (11) with a discrepancy above what is acceptable. However, the data obtained are very close to that found by Millikan, therefore the results are acceptable.
It is advisable to take as much data as possible during the experimentation, in this way the error will be less and if possible for the reader to use programmed tools to extend the data which will reduce the work when calculating the necessary values.
(1) Serway, Raymond y Jewett, Jhon. Física para ciencias e ingeniería con Física Moderna. Volumen 2. Edición: 7ma. 2008. Mexico. Editorial: Cengege Learning. PP: 709-710.
(2) University of Illinois. Messurement of the Electronic Charge by the Oil Drop Method. Illinois. USA. 2009. PP: 2-11.
(3) Parker, Sibyl P. Diccionario McGraw-Hill de Física. 1991. México. Editorial: McGraw-Hill. Tomo II. P: 245.
(4) Fernandez, Braulio. Laboratorio avanzado de Física, un manual para estudiantes de laboratorio del ultimo año de la licenciatura en Física. Edición: 1era. 1991. Mérida, Venezuela. PP: 8-10.
Interesting work, and very good presentation. Congratulations
Thanks very much, I'm glad that you liked it
This is very well explained. I know my comment is not very intelligent, but I just wanted to let you know :)
It makes me feel very happy and honored that you say that my publication is of good quality, thank you very much @lemouth :D
I remember the Milikan experiment as being potentially tedious but quite entertaining from my lab courses ^^
I did this experiment 4 years ago and it took us all day in the lab to get measurements, there were more than 500 measurements and the first 3 times the results were not what I expected due to the bad calibration of the equipment, and I stayed all week in the lab until you get a value close to the charge of the electron
Oh wow
But once you are set up it's remarkable to have such a direct observation in comparision to other experiments. In the end it is just looking through a magnifying glass :D
Exactly after you calibrate the team well the results are wonderful as explained in the post
Excellent work my friend @carloserp-2000. Voted. Congratulations and regards.
Thank you very much friend, it makes me very happy that you like my work