separation of constituents of a mixture in lab
Extraction is a process in which one or more components are separated selectively from a liquid
or solid mixture.
Important applications of extraction procedures can be found in:
chemical industry (anorganics, organics)
chemical metallurgy (extraction of metallic salts from diluted brines)
nuclear technology (reprocessing of nuclear fuels)
environmental technology (waste water treatment)
food technology (edible oil refining)
pharmaceutical industry (extraction of agents)

http://scanamcorp.com/companies-represented/ft-technologies/ies/- Liquid-liquid extraction:
Liquid-liquid extraction is a separation technique in chemical engineering.
Unlike the distillation operation, the extracted product does not change phase, a binary mixture which is to be separated is contacted with a third immiscible liquid called solvent and retained for its ability to extract preferably one. elements of the mixture.
This operation, common in the chemical industry, allows to separate products with boiling temperatures very close but with different physicochemical properties.

http://alatlabsurabaya.com/product/separating-funnel-corong-pemisah/After the operation, two separated phases are recovered by decantation.
Separation of benzoic acid and 2-naphthol:
benzoic acid C6H5COOH
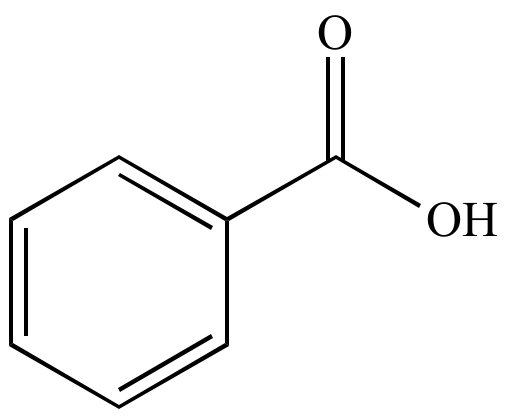
http://urlwww.chem.ucla.edu/~harding/IGOC/B/benzoic_acid.html
it is an aromatic carboxylic acid derived from benzene, and is considered a carcinogenic additive; above 370 ° C it breaks down to form benzene and carbon dioxide.
Benzoic acid has a strong odor and is easily flammable.Napht-2-ol (C10H7OH):
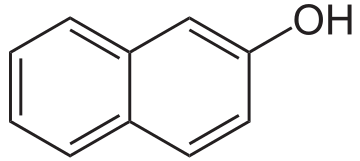
http://www.wikiwand.com/vi/2-Naphtol
it is a bicyclic aromatic compound derived from naphthalene, it is in the form of a colorless crystalline solid.ethyl acetate:
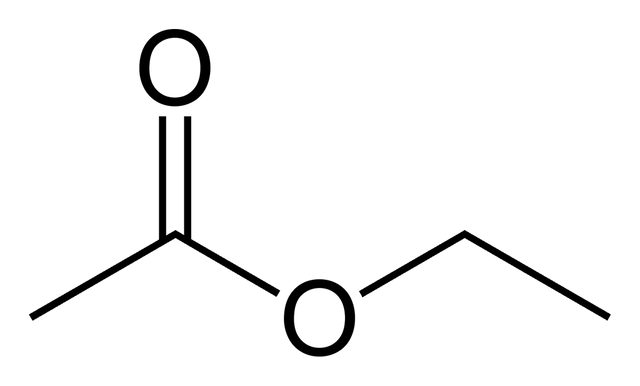
https://fr.wikipedia.org/wiki/Ac%C3%A9tate_d%27%C3%A9thyle
it is an ester resulting from ethanol and acetic acid used primarily as a solvent.Sodium hydroxide NaOH:

https://www.lesamesfleurs.com/products/naohNote:
using the solvent because it has the property of extracting other substances without causing chemical modification of these substances.
The interaction equations:
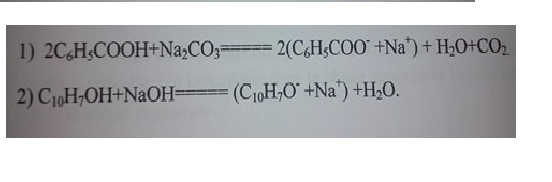
Operating mode:
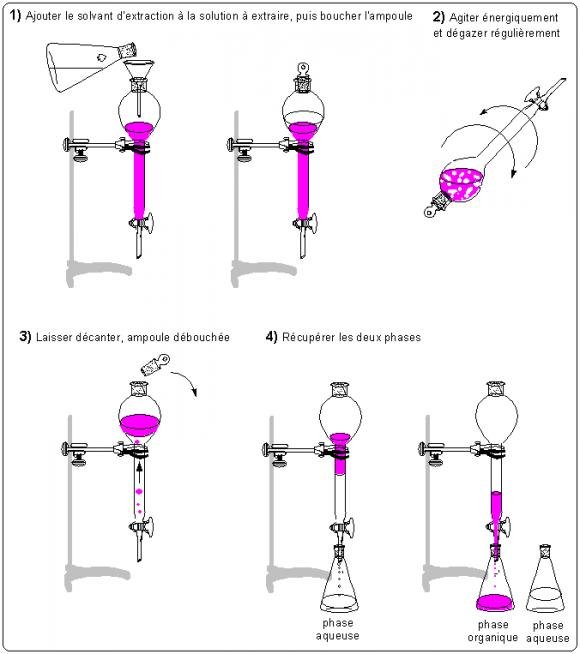
http://culturesciences.chimie.ens.fr/content/lextraction-liquide-liquide-891
1- Prepare the reaction mixture formed of different chemical species
2- pour the solution into a separating funnel
3- add about 10ml of NaCO3 solution
4- Do the degassing to avoid overpressures or depressions
5- close the tap, shake vigorously and quickly and open again (several times)
6- replace the bulb on its support and remove the cap
7- after a few moments that the decantation is done by collecting the lower aqueous phase in a clean Erlenmeyer and extract 3 times the organic phase with 10ml of Na2CO3
8- Transfer the organic phase containing alpha naphthol to a separating funnel
9- Collect the aqueous phases in an Erlenmeyer flask.What is the gas released?
the carbon dioxide begins to evolve in the degassing step, and after that there is the benzoic acid in the aqueous phase and the alpha naphthol in the organic phase.
Réferences:
http://pilodist.de/technology/separation-by-extraction/
http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch15/separate.php
http://www.chem.ucla.edu/~harding/IGOC/B/benzoic_acid.html
https://pubchem.ncbi.nlm.nih.gov/compound/benzoic_acid#section=Top
https://chimie-pcsi-jds.net/polycopies/tp07.pdf
https://fr.wikipedia.org/wiki/Ac%C3%A9tate_d%27%C3%A9thyle
https://en.wikipedia.org/wiki/Extraction_(chemistry)#Modern_variations_and_Applications
This is the best post on Steemit. I gave you a vote now, ya heard?
lol thank you for finding time to read this post, i appreciate that :)
This is a detailed post..... I need you to source your images properly image sorce
Note when doing that, ensure you first click the picture and source only the image.....ensure to make changes
there is links of image sources