The Foundation of Organic Chemistry
Alkanes
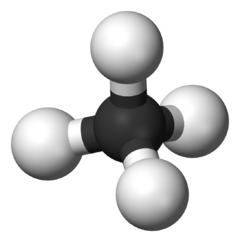
A Molecule of Organic Chemistry
In the world of chemistry, there exists a branch of study termed organic chemistry. Organic chemistry relates compounds that have the basic constitutes of carbon with branches of hydrogen and often in combination with the other main elements such as oxygen, nitrogen and so on. Under this branch of chemistry, a number of subtopics developed when researched by scientists through history. Once such topic is the study of Alkanes, which is often referred to as the scaffold for organic chemistry, as all other topics of organic chemistry originate from an Alkane.
To understand what an alkane is, you must first understand certain preliminary information as well as certain properties of organic chemistry. When a compound chemical composition consists primarily of hydrogen and carbon only, that molecule is referred to as a hydrocarbon. In relation to a hydrocarbon, a molecule that is purely saturated with hydrogen atoms only, is named alphatic alkanes. This term originated from the Greek word aleiphar which directly translates to oil. An important feature to identify all organic molecules, and not just alkanes, is by identifying that hydrogen atoms are located on the surface of all organic materials, as hydrogen atoms form only a single covalent bond, due to the two electrons in its valence shell. All alkanes have a general formula, in which the molecular formula can be identified as:
C(n)H(2n+2)
where n= 1, 2, 3, 4, 5 ….
The first 10 members in the alkane series of a straight-chain are:
Many alkane products from the above list are used widely across the world. Alkanes are extremely versatile as they exist in all three stages of matter which are gaseous, liquid or solid. Methane is an important alkane due to it been a colourless and odourless gas, but also due to it’s responsibility of being the key component of natural gases as well as its heating abilities in important household appliances, such as heaters and stoves. Not only does methane play an important role to us on Earth, it also please an extremely vital role in our solar system. The atmospheres on the planets, Uranus and Neptune (as well as the dwarf planet known as Pluto), contain a considerably large amount of methane. Had methane not been present in these atmospheres, our knowledge of the solar system would have been extremely different, for either the good or the bad. Propane is often identified as the key component of commercially bottled gas, which is used in homes for heating and cooking, and is often distributed as a substitute for natural gas, in areas where natural gas is not easily accessible. Butane is best identified for its extensive applications in lighters as well as fuel canisters that are used for gas camping stoves as well as handheld lanterns.
It is important to note that alkanes with a range of between 5 to 12 carbon atoms, within a single molecule, are often found in the gasoline industry, as these alkanes are found in the chemical composition of petroleum. Alkanes that consist of a different carbon composition (those greater than that of 12 carbon atoms) have roles in other industries.
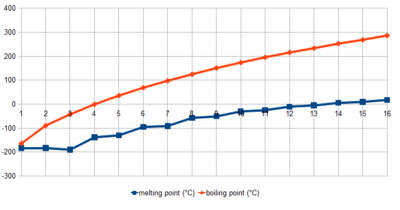
Properties of Alkanes
The reason for the different applications of alkanes are due to the differences of certain properties of the different groups of alkanes. The application of alkanes depends upon properties such as,
- Boiling point
- Melting point
- Branched or unbranched (straight chains)
- Chain length
- State of matter at room temperature, and so on
The polarity of hydrocarbons are not included in this list, as all alkanes are non-polar, due to the fact that the electronegativity differences between that of carbon and hydrogen do not greatly differ. This is an important property of alkanes as this makes them insoluble in water, but alkanes also portray the unique property of being soluble in other non-polar solvents.
Other properties such as the melting point and boiling points of alkanes are determined by the intermolecular forces known as London dispersion forces. It can be noted that scientists noticed a trend in the dispersion forces as per the increase of the alkane molecule. They noticed that the larger the alkane molecule is, the dispersion forces of these alkanes become more attractive. Due to this reason, it can subsequently be stated, that hydrocarbons with a larger molar mass, tend to be less volatile than that of hydrocarbons with a smaller molar mass i.e. hydrocarbons of a shorter parent chain are more volatile than that of hydrocarbons with a longer parent chain. It can also be stated that at room temperature, hydrocarbons with a low molecular weight are gases, hydrocarbons with a moderate molecular weight a liquid and hydrocarbons that have a large molecular weight are solids.
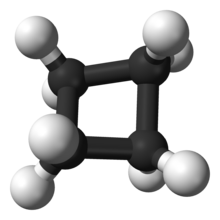
Hydrocarbons are noted to not only be of straight or branched chains, but also can sometimes form rings or cycles. These forms of hydrocarbons are referred to as cycloalkanes. Cycloalkanes are synthesized by the loss of 2 Carbon-Hydrogen bonds, and the formation of a Carbon-Carbon bond. Scientists once again generalized a molecular formula for cycloalkanes as:
C(n)H(2n)
where n= 1, 2, 3, 4, 5 …
Cycloalkanes are used extensively in many industries due to its favorable ability of being more reactive then that of its complementary alkane. The reason for it’s reactivity is related to the ring strain of the cycloalkane. The magnitude of ring strain is known to increase as the ring size get smaller then that of a reference cycloalkane. To illustrate this point, let us consider cyclopropane. Cyclopropane has a bond angle of 60 degrees, thus forming an equilateral triangle. Due to this angle been decreased in comparison to that of straight-chain propane, this form of molecule is more reactive than propane. Cycloalkanes, that consist of a chemical composition containing between 3 to more than 30 carbon atoms, are identified by scientists.
Organic Chemistry
This brings us to the end of my introductory post to the world of organic chemistry. I hope you have gathered the importance and versatility of alkanes and now understand what an important role hydrocarbons play in our everyday society. In my following posts, I will be further elaborating on the other aspects of organic chemistry as well as going through their individuality.
Images are linked to their sources in their description
The End
References:
[1]https://www.sciencedirect.com/topics/neuroscience/alkanes
[2]https://chem.libretexts.org › ... › Map: Organic Chemistry (Smith) › Chapter 4: Alkanes
[3]https://en.wikipedia.org/wiki/Cycloalkane
[4]Chemistry,The central science: A broad perspective
[5]https://www.chemgapedia.de/.../en/.../alkane/alkane.../alkane/verwendung.vscml.html
[6]https://www.cliffsnotes.com/study-guides/chemistry/chemistry/organic.../hydrocarbons
Very good and interesting post