Introduction to Thermodynamics. The energy does not create or destroy It only transforms
Thermodynamics
Is defined as the study of energy, its forms and transformations, as well as its interactions with matter. Thermodynamics is the discipline that within the mother science, Physics, deals with the study of the relationships established between heat and the rest of the forms of energy. Among other issues thermodynamics is concerned with analyzing the effects produced by changes in magnitudes such as temperature, density, pressure, mass, volume, in systems and at a macroscopic level. The basis on which all studies of thermodynamics hover is the circulation of energy and how it is capable of infusing movement.

The availability of energy and the ability of people to use that energy in a useful way has transformed our society. Just a few centuries ago, the majority of the population struggled to survive producing food for local consumption. Currently, in many countries a small fraction of the total labor force produces abundant food for the entire population and many of these people are free for other activities. It is possible to travel great distances in a short time by choosing transport (including both trips to Earth orbit and our nearest natural satellite, for example); communication is possible instant with people anywhere on earth; we have the means to control large amounts of energy at our personal whim in the form of automobiles, power tools, appliances and conditioning the welfare of homes. As a result of the development of science and thermodynamic applications, the ability to obtain energy, transform it and use it to satisfy the needs of our society has grown, changing it from an agrarian to a modern society. In the definition of thermodynamics it is clear that this science is not only useful to engineers in their professional lives but has played, and continues to do so, a vital role in the development of society.
Applications of Thermodynamics in Engineering
Given its generality, thermodynamics is the basic science that serves as a starting point for the study of many other engineering topics; The most obvious is the transfer of heat, which refers to how energy passes from a material or from a place at a certain temperature, to another material or to another place at a different temperature.
Fluid mechanics refers to fluids in motion under the action of external forces and to the transformations of energy between the mechanical and thermal forms during such movement; many topics of material science, such as those that consider the relative amounts of various structural shapes of the materials present in the solids and the way in which these relative amounts they change in different conditions; and, in a certain way, all the topics that refer to energy in any of its forms. Another way to observe the scope of thermodynamics in the studies that interest engineers is to examine their many and diverse fields of application. These include power plants (fossil fuels, nuclear fission, nuclear fusion, solar, geothermal) machines (steam, gasoline, diesel, stationary and propulsion gas turbines, rockets); conditioning of air and refrigeration systems of all types; ovens, heaters and chemical process equipment; the design of electronic equipment (for example, avoiding the overload and failure of individual components, boards with larger circuits and assemblies, as well as understanding the chemical behavior of semiconductors).
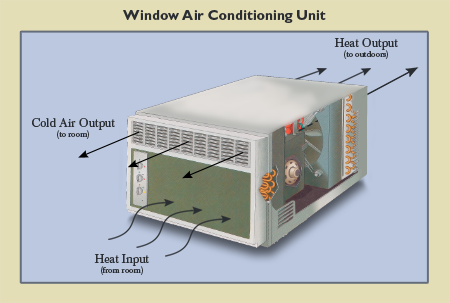
Energy is not destroyed or destroyed but only transformed
The law of conservation of energy states that the total amount of energy in any isolated physical system (without interaction with any other system) remains unchanged over time, although that energy can be transformed into another form of energy. In short, the law of conservation of energy states that energy can not be created or destroyed, it can only change from one form to another, for example, when electrical energy is transformed into heat energy in a heater.
The basic principle of conservation starts from two important assumptions. The first one refers to the fact that energy is something that is "contained". A definite system "has" energy. The second assumption is that there must be a well-specified system that contains that energy. To apply the principle of conservation of energy, the user must define the space or material of interest that "contains" that energy.

For more information consult the bibliography or links that I leave here:
https://www.grc.nasa.gov/www/k-12/airplane/thermo.html
https://www.khanacademy.org/science/chemistry/thermodynamics-chemistry

Resteemed your article. This article was resteemed because you are part of the New Steemians project. You can learn more about it here: https://steemit.com/introduceyourself/@gaman/new-steemians-project-launch