Why do we see different colours?
In everyday life, we see different things around us. The Sky is blue, roses are red, trees are green (or are they?). These colours give pleasure to the eye(just remember black and white TV, real life in black and white etc.). You may have wondered, what imparts colour in a substance? Is it an inherent property of a substance? Does perception of colour vary from person to person as in figure given below? I will try to address those questions in this article so stay tuned.
At first, let me tell you about the general process of sight. The light is sensed by the retina of an eye. It is in the back part of the eye and contains cells. The cells are photo-receptors(Photo means light) and are of two types; rod cells and cones cells. Cone cells are mainly responsible for colour vision and especially sensitive to Red, Blue and Green. So, these colours are called primary colours.
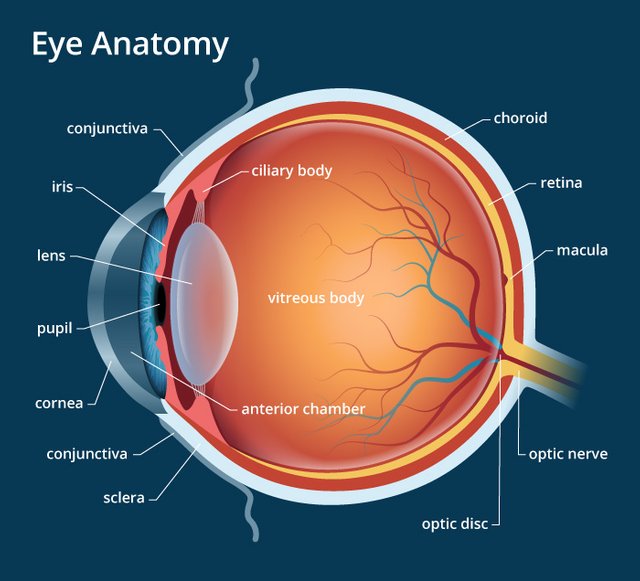
(http://cdna.allaboutvision.com)
Actually, there is no such thing called colour. It's just a name given to indicate how particles behave with ordinary light. We know that ordinary (white) light is composed of seven colours (VIGBYOR). Roses are red in colour this means the white light is incident on roses, it absorbs all colours of light except red. So, the red colour is perceived by our eyes and we see it red. Similarly banana is yellow because it absorbs all wavelength of light except yellow. If a body reflects all wavelengths of light, it is seen white and if it absorbs all wavelengths of light, we see it black.
Let me be little more technical, and try to explain Crystal Field Theory. This theory explains about the origin of color in coordination compounds. Consider there are 5 d-orbitals mainly A,B,C,D,E(actually its different) in the central atom of compound. Orbitals are regions in which electrons are found. Let A,B,C(set 1) be in less energy level. D,E(set 2) be in high energy level.
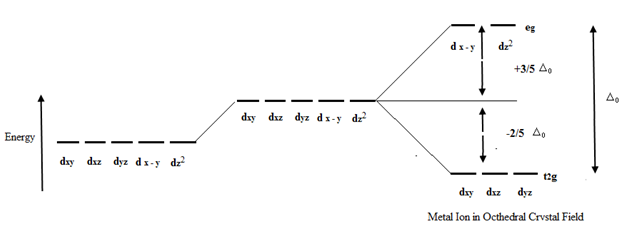
(https://files.askiitians.com)
In ordinary time, electron tries to be in lower energy level i.e. A,B,C(set 1). When light falls in it, light is transmitted, reflected and absorbed. When electron get energy due to absorption, it goes to D,E(set 2). Eventually, it has to come down. When coming down from Set 2 to Set 1, some energy must be released. That energy is in the form of radiation. The wavelength of radiation determines color of the light and hence color of vision.
References

Congratulations @gauss01! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOPCongratulations @gauss01! You have received a personal award!
Click on the badge to view your Board of Honor.
Congratulations @gauss01! You received a personal award!
You can view your badges on your Steem Board and compare to others on the Steem Ranking
Vote for @Steemitboard as a witness to get one more award and increased upvotes!