A recipe for water
Today I am excited to talk about water!
Why is water so important? H2O (its formula) is not just the only chemical formula kids remember from school but it's also the matrix of life. So by understanding what is water and how it came into being you can better understand what you are composed of.
So let's freeze some water into a block ice and with our finest blade we start cutting it (I will assume we are doing this during winter in Wisconsin, so no way the ice will melt). If we keep cutting, eventually we will reach a point in which you will have clusters of 3 atoms (two hydrogens and one oxygen). What does this tell you?
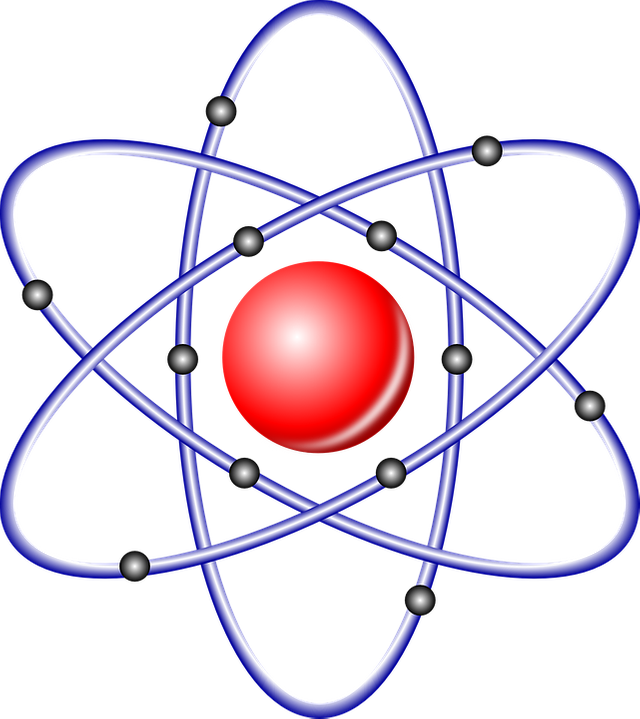
Water is a compound, but it's so important for life that for centuries we believed that water was an indivisible element (I'm sure this news has to reach many people yet). This means that we will never talk about a water atom but of a water molecule. So if we were to make water from scratch we would need some atoms (hydrogen and oxygen) and to make atoms we would need protons and electrons.
Now I hope you will forgive me the analogy (since I'm Italian I should be entitled to use at least culinary ones), but protons and electrons are like knives and forks on a dinner table - it doesn't matter how big is your table, you will always have the same number of each. So each element is like a table, each will have a full set of knives and forks but the number of sets will be different between each tables (each atom has a full set of protons and electrons but the number of sets will be different between each element). Now since I don't personally know any chemist I think I could safely say that chemistry is as simple as counting, without risking of being murdered (chemists might have wanted to murder me already anyway for comparing the periodic table to a messy restaurant full of tables of different sizes).
So if we were to count we would see that the hydrogen atom has 1 of each (1 electron and 1 proton) while oxygen has 8 of each. Unfortunately, only counting would not explain why we need to mix 2 atoms of hydrogen and 1 of oxygen to obtain water, or why sodium (11 protons) is a reactive and soft metal while chlorine (17 protons) is a corrosive gas. That's because chemistry is also about how electrons are deployed.
The electrons in fact, are definitely not a static entity, they rather swarm around the nucleus in regions called orbitals, a bit like the bees around a hive. So chemistry is also about how all the available electrons in an atom are distributed among all its orbitals.
Within an atom there is no gravity, but all its components are held together by electrostatic forces where the electrons are the negative and the protons the positive. However, electrons can be stripped away from atoms, when this happens, the atom will have a positive charge because it will temporary hold more protons than electrons and it will also be named "ion". That is also why the number of protons in an atom is very important and determine its properties. In turn, protons are not so easily moved, if you were to dig them up from the nucleus you would require a lot of energy and you will end up turning your atom into a different element.
There is another player in the atom, a particle with roughly the same mass of a proton but without any charge, in fact we call it "neutron".
Generally, heavier atoms will have more neutrons so while we have almost the same ratio neutron to protons in carbon, oxygen and nitrogen, we will have for example 40% more neutrons in lead relative to its protons. However, sometimes even atoms of the same element can have different number of neutrons, for example:
- Oxygen can have 7 or 8 or 9 or 10 neutrons with his 8 protons
- Hydrogen can have 0 or 1 or 2 neutron, but generally 99.99% of the hydrogen atoms have zero neutrons. When hydrogen has neutrons it's called heavy hydrogen or deuterium.
I am sorry to have enforced all this chemistry on you but if we are to cook up water we need to know all our ingredients, the good news is that we can start cooking pretty soon, we just have to cause a big bang and then we are ready.
Universe
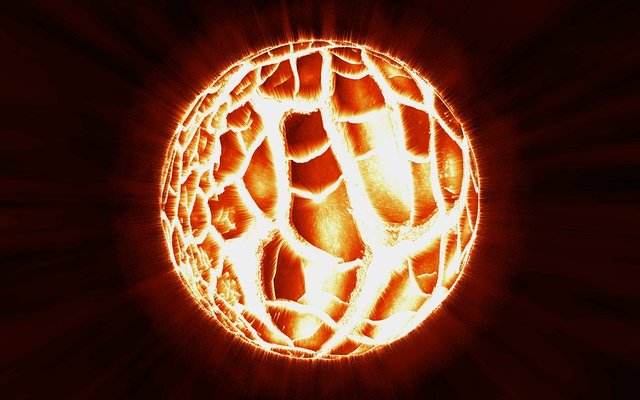
Once upon a time, all the matter in the universe used to be amassed in a tiny volume, but then the universe went bang. Today we only see a dim and cool remnant of what once was a cosmic fireball. A millionth of a second after the big bang marked the birth of time and the universe. With them, we got a temperature of one trillion degrees celsius and a cosmic soup of protons and electrons. To make things more spicy, the soup was also seasoned with neutrons and neutrinos and simmered in a broth of X-rays. However, this temperature was simply too high for electrons and protons to hold onto each other, so for a while nothing much happened. So we had to wait for a whopping 100 seconds for the temperature to start dropping to about 3 billion degrees, just enough to enable the stronger interaction between particles to occur. For example, because the force that binds protons and neutrons is incredibly strong, they started combining right away and started forming the heavier elements. At 4000 degrees celsius, protons could team up with electrons and start forming hydrogen atoms.
Crafting atoms
If it was just for the big bang, we would have a very short periodic table composed of just a very small list of elements. However, in this cosmic cloud of gas there were areas slightly denser than other. This heterogeneity caused a minimal attraction force that gradually become more accentuated and started condensing matter into compact blobs, and so gravity was born.
Over time, gravity got stronger and its pulling force was causing stuff to heat up again until it started blazing. Today we call these fireballs "stars". And these are the true engine of creation, the alchemists of the universe responsible for crafting and converting elements.
In fact, in the fiery hearts of the stars, nuclei of hydrogen were fusing with heavier elements fueling a process called nuclear fusion. However, the mass of elements is not the whole multiple of the atomic weight of hydrogen, this is because elements exist as a mix of isotopes but also because a tiny fraction of the mass is spent to release this huge amount of energy, the missing mass could be calculated using the famous formula: E = mc^2 .
By the way, eaerlier I mentioned that gravity is exerting a pulling force within the stars, so why don't they just collapse? That's because of the radiation produced by the nuclear fusion gives the star buoyancy. But let's not get derailed off topic.
At this stage, the star is burning helium to fuel its atom crafting activity. So nuclei fuse to form oxygen, carbon etc..For the sake of simplification let's skip a few steps but let's just say that the star starts burning whatever it has left producing different temperatures and crafting different elements in the process. Also the size of the star will dictate also its destiny, large stars in fact, end their life in a remarkable way, with a bang. At some stage they will become supernovae and explode. Obviously such a tremendous amount of energy crafts even more elements such thorium and uranium that go on enriching other elements. So these explosions sprinkle the void between other stars with debris, this is relatively speaking, as our planet was once probably part of this description.
So to recap, the big bang triggered the formation of elements, the 3 most common elements that formed were in order of abundancy:
1) Helium
2) Hydrogen
3) Oxygen
Considering that helium is a cosmic loner, an unreactive element, it's not surprising at all that the union of hydrogen and oxygen, the universe's most popular reactive elements, spawned something so special like water.
So water is the result of 2 incredible cosmic acts of creation:
- the big bang that created hydrogen soups
- and the stellar alchemy that turned hydrogen into many other elements
Water!!
Obviously the ancient Greeks knew it! They guessed that our planet originated from chaos. Etymologically chaos means "gas". Sometimes it's almost scary to see how much the Greeks knew!! However, talking about gas, today we know that the atmosphere of our planet was completely different upon its formation. In fact, in the pre-biotic earth we had plenty of gasses such as neon, argon and krypton, these are gasses chemically un-reactive thus we would have expected them to have persisted on our planet until our days. But, we must consider that our planet in its infacy was not exactly a resort. In fact, most likely there were showers of celestial bodies on our planet and the impact probably created a global inferno. Scientists think that the earth probably even collided with a celestial body the size of Mars. The impact and the amount of energy released must have been massive, so big to boil away all the atmosphere, leaving our planet as a bald ball of magma for millions of years. I can safely say a global nuclear war would probably sound like a fart in comparison. By the way the impact also probably chipped away a large chunk of our planet, forming the moon.
Fast-forward to about 4.5 billion years ago, our planet started cooling off and all its elements started coagulating. Obviously the heaviest elements tended to sink toward the core of the planet and the lightest elements formed a rocky crust. Magma also dissolved many compounds releasing vapours in a process called degassing. The earth gravitational field was not strong enough to retain some of these gases and light elements like hydrogen simply diffused away. Even today our planet continues to steadily lose water and hydrogen but very slowly. In fact, in our upper atmosphere there is a process called photolysis in which ultraviolet rays from the sun split water into oxygen and hydrogen. With the hydrogen just drifting into space. The cost of this the equivalent of a lake's worth of water per year. If this was to come from a single lake it would be a lot, but obviously, by averaging it over the whole planet is not very much at the end. Since our planet has formed we lost only 0.2% of our water reserves via photolysis.
So these were steamy times for our planet, there was an incredible amount of water in the sky. When the temperature of the earth cooled enough, the water vapours condensed and our sky must have literally have been raining down, Biblically speaking it must have been an universal deluge. The only difference is that instead of eradicating life from our planet it set the stage for its entry.
Well it took just a few billions years but at the end we managed to make some water. I realize this post must have been long and heavy to digest, even if we were making soups :)
About this, after setting tables and talking about soups I guess it's time for me to cook dinner, I hope you enjoyed this post, if you did please up-vote it and leave a comment, it means a lot for me. Obviously there story doesn't end here, if I see enough interaction I may continue this tale.
Sources:






Wow, this is a lot of information. Water is a lot more than i thought it was. I'll have to reread to assimilate all the information. Thanks for sharing
Thanks for stopping by, yes it's a heavy soup to digest :)
I wanted to thank everyone that voted for my post especially @steemstem and @curie if you don't promote yourselves when you vote I will do that for you. SteemSTEM is a cool project that supports science on Steemit, you should check it out! To get in touch with the SteemSTEM team you can visit their channel: https://steemit.chat/channel/steemSTEM

This post has been ranked within the top 25 most undervalued posts in the first half of Nov 25. We estimate that this post is undervalued by $19.64 as compared to a scenario in which every voter had an equal say.
See the full rankings and details in The Daily Tribune: Nov 25 - Part I. You can also read about some of our methodology, data analysis and technical details in our initial post.
If you are the author and would prefer not to receive these comments, simply reply "Stop" to this comment.
All the glory to the Lord who made it. The Lord provides man and animal with water. If there was no water there was no life.
God is literally a star!!