Let's Learn Anatomy!!! #2 - Atoms, Molecules, and Chemistry
Pop quiz! How many atoms are in the universe? 100 trillion? 100 quadrillion? 100 quintillion? Not even close. The best estimates are somewhere on the order of 10^80. That's an insane number, and it might even be conservative.
Just to illustrate how large of a number that is, when written out it looks like this: 100,000,000,000,000,000,000,000,000,000,000,000,000,000,000,000,000,000,000,000,000,000,000,000,000,000,000. For a comparison, the number 100 trillion looks like this: 100,000,000,000,000.
Damn. And here I am blown away when there's a $1,000 in my bank account...
While this may not be a physics or chemistry class, realizing that these sciences are part of the bedrock of biology is of the utmost importance. Even though we won't be discussing in detail the immensely complex biochemistry that is the human body, we still need to understand that at the most basic level, physics and chemistry are the reality. Biology, and therefore anatomy are said to be emergent properties of chemistry, meaning that when the chemistry becomes sufficiently complex, biology blossoms from the chaos and now has it's own set of governing rules that are not part of the underlying chemistry's rule set.
To be honest, it's definitely possible to speak about and learn anatomy without knowing the physics or chemistry involved, but there would always be a slight gap in understanding. Now, this will by no means be comprehensive, and I will only speak in terms of the extreme basics. However, we'll see that this will be more than enough to get us through our anatomy careers.
Disclaimer! I am not a chemist, nor do I pretend to be one. These basic concepts are meant to be just that: basic. There are going to be multiple ways to clarify and be even more precise with the topics I bring up, but note that I am not going to be able to speak to every little nuance with these subjects. Each one deserves its own separate post to fully describe them in detail. I will save that for another day.
Behold! The Mighty Atom!

Credit: http://wonderopolis.org/wonder/why-do-atoms-form-molecules
Considering this isn't a history lesson, and you probably are already debating skipping through this hoping to catch the necessary bits of info, I'll be brief with the background of the atom.
The idea for the atom goes way back to ancient Greece, where philosophers postulated the existence of something so small, you couldn't possibly cut it any smaller. In fact, the name atom comes from the Greek word atomos, which means indivisible. While an amazing idea that was far ahead of its time, it also happened to be wrong on several accounts. For instance, you probably already know that we can indeed split an atom into smaller parts (quarks), which pretty much throws out the entire basis of the idea. Despite this, the name stuck: Atom.
Atoms come in all shapes and sizes, but share commonalities with one another that are important to mention. Each atom contains a nucleus, which consists of nucleons called Protons and Neutrons. Protons are positively charged, while neutrons, like its name suggests, have a neutral charge. Protons and neutrons have basically the same mass, and usually come in similar amounts to one another. For instance, if you have one proton, you should usually have one neutron. However, neutron count can vary, and when the numbers no longer are the same as a proton, you now have what's called an isotope. Isotopes are the reason you may have heard certain elements spoken of with a number after it, like Carbon-14, or Carbon-12. Both are carbon atoms, but are also different isotopes.
The Electron
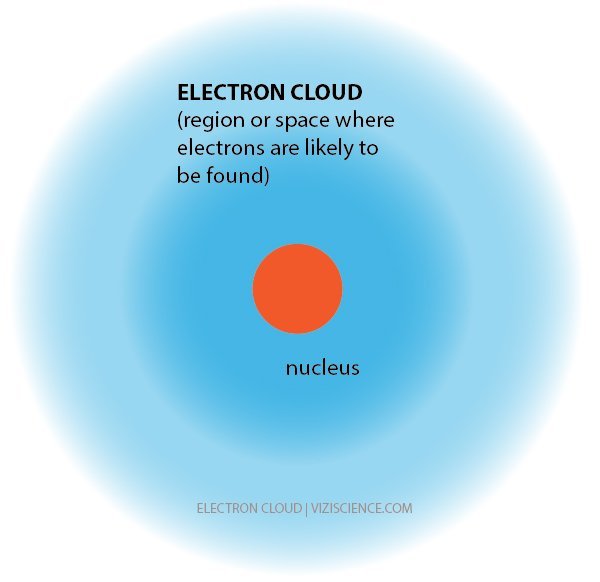
Credit: https://viziscience.com/chemistry-energy-and-bonds/electron-cloud/
Electrons, the negatively charged counterpart to the proton, are endlessly fascinating to me. First of all, they're tiny. So small in fact, scientists don't even know how small it is. That's pretty damn small. If you were to compare the mass of an electron to the mass of a proton, a proton is somewhere around 1800 times as large, and a proton is already small as it is.
Next, electrons, like all particles, display a wave-particle duality. This makes it impossible to ever lock down it's literal position at any given time. Instead, we look at all of it's possible locations and then determine where it's most likely to be. This is what we call the electron cloud.
For years we were thinking and teaching about electrons all wrong. You may have originally learned about electrons "orbiting" the nucleus of the atom, similar to how the planets orbit our Sun. This was called Bohr Model, and for quite some time it was considered to be accurate.
But as most things with science, when more evidence is found suggesting a different explanation, things must be revised and updated in order to provide the most accurate description of reality. Electrons can only be described as where they are most likely to be, not where they actually are.
How poetic!
It's Like an Eggshell.. Except Not..
How many electrons can an atom contain within it's boundaries depends on the atom. While electrons may not be whizzing around the nucleus like we originally thought, they still occupy the space outside of the nucleus in specific numbers. These are called electron shells.
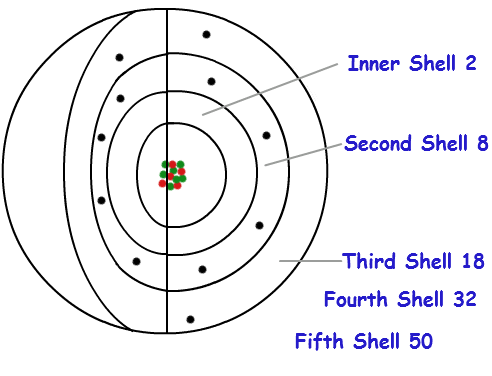
Credit: http://chemistry.tutorcircle.com/inorganic-chemistry/electron-shells.html
Electron shells are basically energy levels with which the electrons can occupy. The first electron shell can contain 2 electrons, while the next shell can contain 8 electrons, with the third being able to hold 18 electrons. While it is technically possible to have an infinite amount of electron shells, the farther out you travel from the nucleus, the more unlikely it is that you'll find an electron. The reason for this is that electrons are attracted to the nucleus of the atom, and prefer to remain as close to the nucleus as possible. While this may not hold true for every instance across the board, it's useful to think that electrons won't occupy a position in an electron shell unless the spaces from the shell closer to the nucleus are already taken.
Another important thing to be aware of with electrons is that there are usually as many electrons in an atom as there are protons in the nucleus. However, this isn't a rule necessarily. If an atom gains or loses an electron, it is now called an ion, and has become a charged particle.
Ions Make the World Go 'Round
Recall that protons have a positive charge, neutrons have no charge, and electrons have a negative charge. If atoms contain the same amount of protons and electrons, the atom is said to be neutral, seeing as the charges cancel one another out. If an atom gains an electron, the atom is now called a anion, or a negatively charged atom. If it loses an electron, the atom is now called a cation, or a positively charged atom.
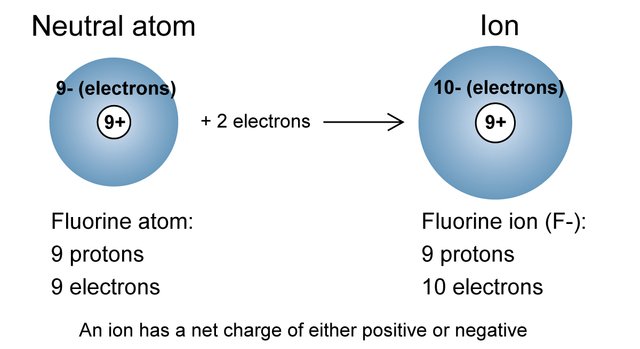
Credit: https://online.science.psu.edu/chem101_sp1/node/6293
Biology loves to use ions in its physiological processes. The reason being that ions act like anything in nature that has a charge, and can become attracted to ions of an opposite charge, or even be repelled by ions of a similar charge. Nearly every physiological process in your body uses this property to its advantage, with one example being the neurons in your nervous system utilizing the cation Sodium (Na+) and the cation Potassium (K+). To be completely accurate, these are more properly called electrolytes.
Brawndo.. The Thirst Mutilator!
I guarantee you've heard of electrolytes before. While the movie Idiocracy added them to their incredible drink Brawndo, Gatorade has been marketing the electrolyte for years as being essential to you hydration and health. So what exactly is an electrolyte?
An electrolyte is nothing more than an ion in water. To be specific, it's something that dissolves into a cation or anion when introduced to a solvent. In the body, this solvent is usually water, or something close to it.
A great example in the body where electrolytes are essential to normal functioning, is the kidney. We'll discuss the kidney and it's plethora of functions at a later date, but for now just think that the kidney is necessary in order to maintain hydration levels. It accomplishes this by shuttling Na+ in and out of various urinary tubes, which happens to attract and bring water with it. That's right, without Na+ and your kidney, you'd be a chaotically unbalanced bag of water and bones. Sweeeeet.
Hold On.. If I Lose an Electron, Where Does it Go?
Great question. I mean, it's not as if they are lost to the cosmos, never to be found again. The electrons have to go somewhere, and that somewhere is probably exactly where you thought it'd go: to another atom. That's right, atoms steal from other atoms. We call atoms who utilize this thievery to their advantage free radicals, and they are not the freedom loving individuals you hope for them to be.
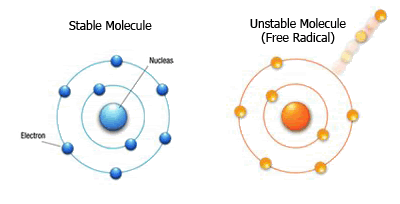
Credit: https://coconutcreamcare.com/2012/08/30/inflammation-free-radicals-and-the-health-benefits-of-nuts/
The easiest way to think of a free radical is as an unstable atom. What makes it unstable? To answer that we need to look back at the electron shells we spoke of earlier.
While I mentioned that there are technically an infinite amount of electron shells that the electrons can occupy, they usually occupy the electron shells closer to the nucleus first, only filling in the more distant shells as necessary. The outermost shell is what is called the valence shell, and the electrons that occupy this shell are called valence electrons. The important thing to understand about valence electrons is that if some type of chemical reaction with electrons is going to occur, the valence electrons are going to be the first to effected.
The issue at hand here is that atoms are considered stable based upon the amount of valence electrons it has in its valence shell. Electrons like to be paired with one another, meaning that if you split them up, they will naturally search for a more stable position with another electron from a different atom. In doing this however, they can create another free radical in the process.
The body creates free radicals all the time, through normal metabolic processes. The heat created in these processes can be enough to break the bonds between atoms, creating two separate free radicals. These free radicals will then move to another atom, steal one of its valence electrons, thereby making itself stable again, but creating another unstable ion in the process. This will continue on until two free radicals meet up with one another and decide to bond, which stops the perpetual stealing.
Based on where in the body this process occurs, different outcomes can happen. If this happened in the nucleus of a cell, the DNA can mutate, causing a whole host of issues for the organism. It could also happen in the cell membrane of the cell, which can cause the death of the cell and can contribute to the aging process.
We will discuss free radicals in an entire post of its own in the future, because there is so much more to say about them, especially when it comes to nutrition. But for now, this will do.
One Step Closer to Really Learning Some Anatomy
We now have all the basic chemistry needed to begin our anatomy careers. I'll be using the terms ions, electrolytes, and free radicals quite often, so make sure you feel comfortable with what they mean and why they're needed.
In our next lesson, we'll be meeting the extremely important macro-molecules called proteins, carbohydrates, and lipids. Pretty soon we'll be piecing a body together, molecule by molecule.
See ya next time!
Hey! In case you want to connect a bit more with the science community on steemit, I invite you into the #steemSTEM channel on steemit.chat (in case you haven't already found it yourself)!
Awesome! Thank-you! I had no idea it existed!
Really nice post. Looking foreward to more.
Id like to nominate the post in a curation group im in
This gem of a post was discovered by the OCD Team!
Reply to this comment if you accept, and are willing to let us share your gem of a post! By accepting this, you have a chance to receive extra rewards and one of your photos in this article may be used in our compilation post!
You can follow @ocd – learn more about the project and see other Gems! We strive for transparency.
Thanks! I would like to accept your offer, and appreciate you sharing!
thank you for your effort to keep science alive :)
Thanks! Science is beyond beautiful to me, so I'm happy to do whatever I can to show that to others
Hello. Excellent post atom
Follow me
I've never heard it referred to as an "electron cloud" I may need to update my chemistry knowledge. Thanks for the refresher!
Thanks! It's basically a full-time job keeping up with the terminology these days, so I know what you mean.
nice post great valuable info
:)
Thanks! I appreciate it!