SILICATE MINERALS
Silicate minerals crystals structure are groupedal according on how silicon sharing oxygen atoms are placed on a planar surface which are grouped into : Tetrahedral and octahedral unit.
The tetrahedral units have four oxygen atoms which reacts with other cations in which three of the oxygen atoms forms the base and the last atom forms the apex of the pyramid when placed on a plane surface.
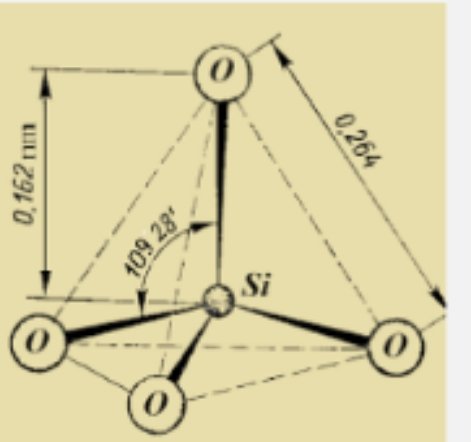
The octahedral unit requires eight oxygen atom to coordinate with other cations to form a eight sided shape when placed on a plane surface.
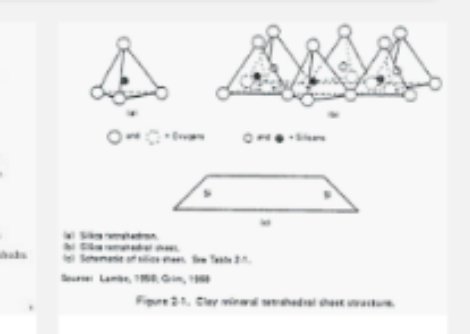
The maim classes of silicate minerals are:
#1. Nesosilicates:They are also called orthosilicates or independent silicates.They do not share oxygen atoms with each other.they dont have the si-o-si bonds .they are susceptible to weathering process because they are unstable.examples are olivines,zircon,garnet

#2.Sorosilicates:They are also called pyrosilicates or double silicates.unlike nesosilicate,they share oxygen atoms and are not susceptible to weathering process.they have the si-o-si bonds
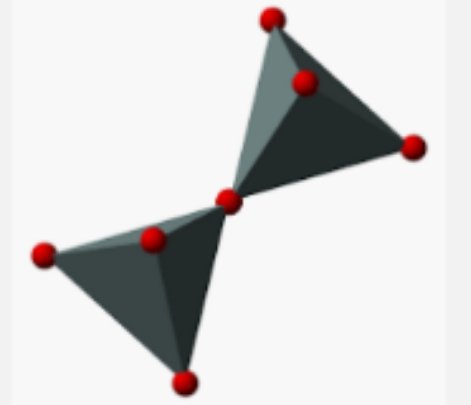
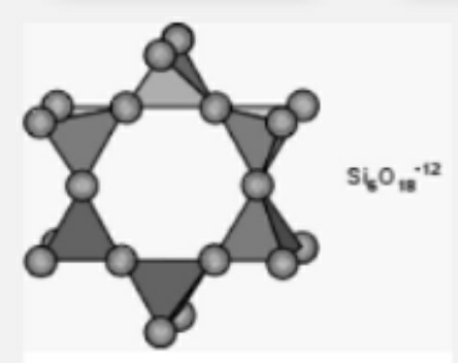
#3.Cyclo silicates: They are also called cyclic silicates or ring silicates.they describe the cyclic pattern of silicates.the rings are 3,4,6,8 but the most common ones are 3 and 6
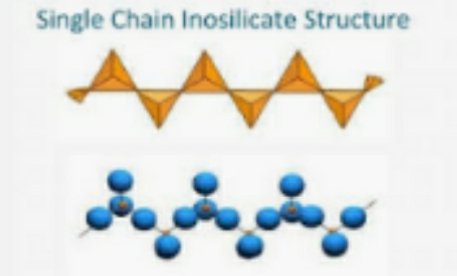
#4. Inosilicates:They are grouped into single chain and double chain silicates.
The single chain silicates shares two oxygen with the adjacent silica.they exhibit isomorphous substitution
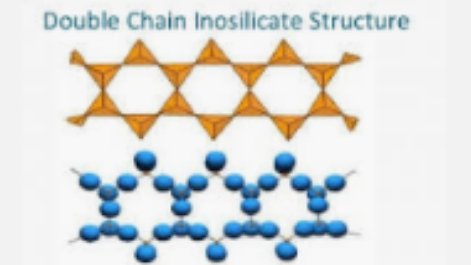
Double chain silicates is formed by joining single chain silicates and exhibit higher isomorphous substitution
Hello! I find your post valuable for the wafrica community! Thanks for the great post! We encourage and support quality contents and projects from the West African region.
Do you have a suggestion, concern or want to appear as a guest author on WAfrica, join our discord server and discuss with a member of our curation team.
Don't forget to join us every Sunday by 20:30GMT for our Sunday WAFRO party on our discord channel. Thank you.