Heat Changes That Can Change An Object Like Water

Heat Can Change The Temperature
When water is heated, water receives heat energy from a flame that is ignited through the boiler that manifests it. Water receives heat energy, characterized by a rise in temperature. The greater the heat energy received by water, the greater the rise in water temperature.Changes In Heat And Temperature
This property shows the greater heat received by an object, the greater the temperature rise in the object. The increase in heat is proportional to the heat transfer from fire to the receiver, and also proportional to the temperature rise. If the amount of heat expressed by Q, and the temperature change expressed by DT / temperature change, then the heat relation with the temperature change can be expressed by the equation:
While based on the SI unit, the energy of heat is expressed with joules (J). Joule comes from the James Prescott Joule experiment, that 1 calorie equivalent to 4.8686 is rounded to 4.2 joules, so 1 joule is comparable to 4.2 calories.
Once we know the temperature change during heating, as one of the factors that influence the heat to raise the temperature of the substance, then from the following activities we will understand other factors.
When heating water, the longer the higher the temperature rises, and the higher the temperature, the more energy it needs. Thus the temperature change affects the amount of heat energy required.
In addition to temperature changes, there are two more factors that can affect the amount of heat energy needed to raise the temperature of the substance. There are two factors:
From the activities we have done, it can be concluded that the time required to raise the temperature of the liquid is influenced by the amount of fluid. The more fluid, the more time it takes to raise the temperature of the substance. Thus, it takes more heat.
The number of heated objects is generally expressed by the mass of the object. The mass of an object is denoted by m, in kilograms (kg). Thus, the amount of heat required (Q) is proportional to the mass of the body or by its equation:

The amount of heat required for each kilogram of substance, to raise the temperature of one Kelvin is called the heat of the species. The amount of heat needed to raise the temperature is proportional to the heat of that type of thing. The heat type is denoted by c, and the amount of heat with Q, then the equation is formed:
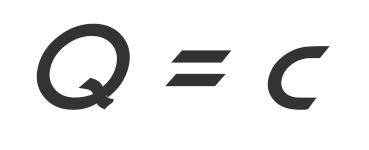
Thus, the heat required to increase or decrease the temperature of an object depends on the mass (m) in kilograms (kg), heat type (c) with units J/kg.K or J/kg °C, and temperature changes (m) ΔT) with a unit of Kelvin or ° C.
The relationship between the amount of heat (Q), the mass of the object (m), the type of heat (c), and the temperature change (ΔT) can be expressed by the equation:

The Conclusion:


About The Science I Have Written:
Science: | Science: | Science: | Science: | Science: | Science: | Science: | Science: | Science: | Science: | Science: | Science: | Science: | Science: |

Congratulations! This post has been upvoted from the communal account, @minnowsupport, by youngky from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews, and netuoso. The goal is to help Steemit grow by supporting Minnows. Please find us at the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
If you would like to delegate to the Minnow Support Project you can do so by clicking on the following links: 50SP, 100SP, 250SP, 500SP, 1000SP, 5000SP.
Be sure to leave at least 50SP undelegated on your account.