[Fun science!] #3. The story of Rutherford and quantum mechanics
Fun science!
The story of Rutherford and quantum mechanics
This article has been translated by @zoethehedgehog
Original article in Korean
#1. The story of Rutherford and quantum mechanics
#2. The story of Rutherford and quantum mechanics
The atomic model we see often
Image Credit : Wikimedia Commons
At first, no one knew the existence of an atom.
When it was found, most people thought it was a hard object.
At 1897, when the electron in the atom was found, J.J.Thompson imagined it would be like a raisin bread.
A: Hm? A raisin bread?B: It’s closer to a watermelon. We should call it the ’Watermelon model!’
The people thought there must be seed-like negative electrons inside a electropositive watermelon-like structure.
But Rutherford proved with an experiment that the positive electricity is not widespread but joined in a very small place.
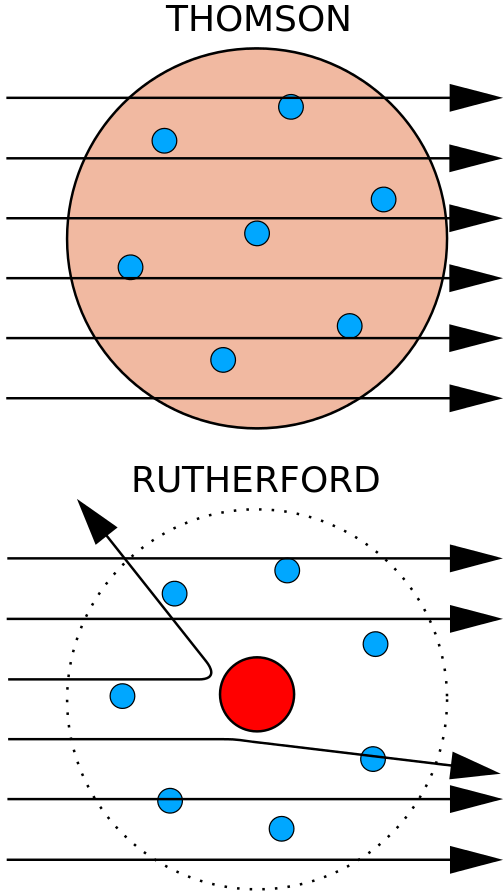
Image Credit : Wikimedia Commons
An empty atom
This model was already suggested at 1903 by a physicist from Japan, Nagaoka Hantaro, but it had been rejected at that time for he had no evidence.

Nagaoka Hantaro, Image Credit : Wikimedia Commons
The model was rejected because if it was real, the electron had to gradually lose it’s energy and become a neutron.
The lost energy has to be accompanied with a light emission.
But this didn’t happen.
The model of Rutherford had the same problem,
but his experiment had evidence that the atom was empty.
Rutherford:
The atom is empty, not like I can do anything about it.
Find the reason yourselves.I’m an experimental physicist :D
At first, Rutherford’s model didn’t get a lot of attention.
But later when Niels Bohr (The one who disputes with Einstein) used it and made an electron orbit model to explain the spectrum of an atom, it became the representative model of an atom.
Rutherford: Thanks Bohr :)
One atom resembles the solar system.
Isn’t it marvelous that such a small world resembles the enormous solar system?
Who knows?
Our solar system could be a small atom of a bigger world.
It’s fun to imagine.
In the movie 'Men in Black', there is a scene where the camera zooms out.
You see the earth, then the solar system, then the galaxy.. Then it becomes a bell on the cat’s neck.
But the representative atomic model is used for the understanding of the atom, and is not how the actual atom looks like.
The electron doesn’t orbit around like a planet, but it’s filling up every space like the blades in a fan.
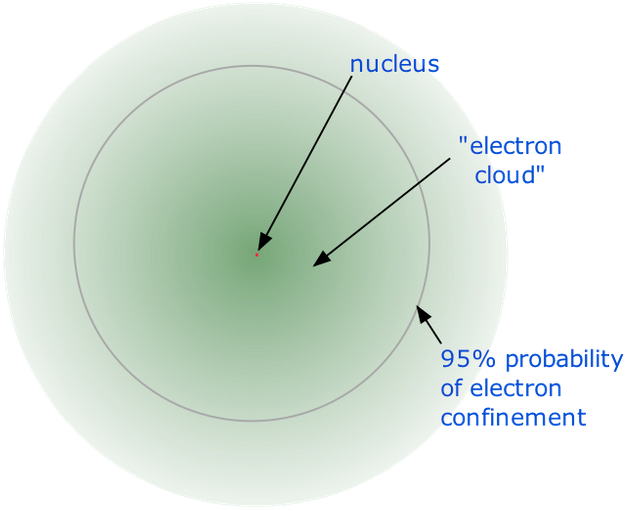
The atomic model, Image Credit : chem.libretexts.org
The unbelievable truth is, the electrons are everywhere in here.
You can find lots of other articles on science on #science.
