Super Cool Science S#!t #13 - SALT!
I can hear it now...
"Sweeney has lost his mind, and thinks he can write a cool science article about salt! "
Give me a shot, will you? After a dozen of these Super Cool articles, I've never steered you wrong, have I? Okay, then. Here we go...

Source
Salt is a delicious ionic compound made up of a highly reactive metal and a poisonous gas.
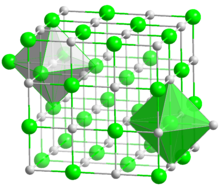
Sodium and Chlorine ions come together in equal parts to form a mineral that is essential to our survival. Forming small, cubicle grains, each ion in the molecule is surrounded by six oppositely charged ions. In the image on the right, the larger Chlorine atoms (green) are arranged in a cuboid form, with the smaller Sodium atoms (silver) filling all the gaps, forming a bond that is so strong, only the most highly polar solvents like water can dissolve it well.
This time of year in the United States, salt is very important for keeping our roadways from icing over. This is possible because salt lowers the freezing point of water, meaning it can't freeze into ice at the normal 32 degrees Fahrenheit (0 degrees Celsius). Salt dissolves into the water, breaking down into its separate elements. This makes it much harder for the water molecules to form a rigid structure, meaning less ice on the roads!
Salt is life
Both Sodium and Chlorine are crucial for life to exist, and are the fuel behind many of the body's processes. They're both required in just the right amounts, as too little or too much can lead to health issues.

Sodium is an electrolyte, a mineral that carries an electrical charge when dissolved in body fluid. Most of the sodium in the human body is found in the blood and extracellular fluid. It helps maintain fluids in the body, and excess sodium is excreted mostly via sweating and urinating. Sodium plays a key role in blood volume, blood pressure, and PH balance Just like anything else, good things are best in moderation.
Too much sodium in your body can lead to high blood pressure. This occurs when an excess of sodium in the bloodstream attracts water to keep a salinity balance. This extra water leads to pressure in the veins, as there is much more fluid flowing through them than normal.
Too little sodium can trigger a couple different processes in the body. First, the adrenal glands will start to produce [Aldosterone(https://en.wikipedia.org/wiki/Aldosterone), a hormone that causes the kidneys to hold on to sodium, and excrete potassium. This can lead to less urinating, and an increased blood volume. Low sodium also makes the pituitary gland to make Vasopressin, which tells the kidneys to retain water.

The ionic form of chlorine, chloride, is also essential to many bodily functions, such as the creation of hydrochloric acid for digestion, and the regulation of body fluids. Most of the chlorine in our bodies is found in the extracellular fluid, while a large portion of the rest is contained within red blood cells.
In its pure form, chlorine is a very toxic gas that attacks the respiratory system, eyes, and skin. It is an oxidizer, stripping other materials of electrons, which can lead to combustion.
Most processed foods contain all the sodium and chlorine we could ever need in the form of salt, so we're in more danger of having too much salt than having too little. The table salt we have in our homes is often iodized, containing trace amounts of another mineral, Iodine.
Salt as an industrial resource
Most of the salt mined in the world is used for chemical production and processing. Here's a likely incomplete list of industrial processes we use salt for:
- Sodium Hydroxide - Used to make paper, soaps, drain cleaners, and more.
- Calcium Chloride - Commonly used as road salt
- Sodium Carbonate - Used in the production of glass, film developing agents, taxidermy, and more.
- Hydrochloric Acid - Found naturally in gastric fluid, also used in the production of PVC and other plastics, gelatin, leather processing, and more.
- Sodium Sulfate - Mostly used in detergents and for turning wood into wood pulp.
Salt is also used to:
- Make and alter drilling fluid
- Cure concrete
- Make a brine rinse for dyeing
- Process metals like aluminum and copper
- Treat leather products
- Preserve meats
- Create certain white plastics
See? Salt is Super Cool!
Want to see something cooler? Salt reacts to many things, sodium and chlorine react to even more... Here's a really pretty science experiment you can do at home with things you can find at your local store. Unfortunately, I did not have the materials to do this myself, but I will collect them, and demonstrate this in an upcoming post series I have planned.
KiwiCo demonstrates on their YouTube channel how to create a "Crystal Tree" using salt and ammonia.
Super Cool Bonus: Click that link to KiwiCo, and get $10 off your first Kiwi Crate! My four year old has been signed up to this for a couple months. We get shipped a monthly "Crate" that's full of awesome STEM activities to help your children grow into critical thinkers!
References:
- https://en.wikipedia.org/wiki/Sodium_chloride
- https://www.britannica.com/story/why-does-salt-melt-ice
- https://www.merckmanuals.com/home/hormonal-and-metabolic-disorders/electrolyte-balance/overview-of-sodium-s-role-in-the-body
Ready to learn more? Check out how Super Cool Glowing Planets are!

I will definitely try this crystal tree :)
Thank you
@boycharlieplays runs an afterschool program and summer camp and last summer one of his counselors did the Christmas tree project except she called it crystal snowflakes .
You really did lay out it all out there about salt, we the One Strong Program greet you @thatsweeneyguy with an upvote and a tip. #steemraisefam
Whoop whoop! #steemraisefam! :D
Thanks @thatsweeneyguy for another interesting and informative post. That crystal tree looks like a great project for me to do with my grandkids.
My older son will love it!
They are home schooled and things like this are great lessons.
Super cool project! The Kiwi Crates are a great idea for homeschooled kids for extra learning! Great post, @thatsweeneyguy!
My four year old starts his homeschooling journey this year, and we couldn't be more excited for things like Kiwico!
Thanks for sharing this mate, it was a good read whilst on the toilet haha :)
Thank you for sharing this informative post @thatsweeneyguy
Thank you for simply scrolling to the bottom of the post and leaving a generic comment 1 minute after I published this ;)
thank you for sharing this. it is a compelet life history of salt
Fascinating stuff!
Excellent post ..
Really nice post! I still remember the exactly same kind of crystal tree was actually sold as a science toy when I was young and I got one on those old days !