Photoelectric effect and photocells
Few days back i made a very informative post about X-rays in which x-rays are produced by exposing a beam of highly accelerated electrons on the metal surface.Click here to read more.
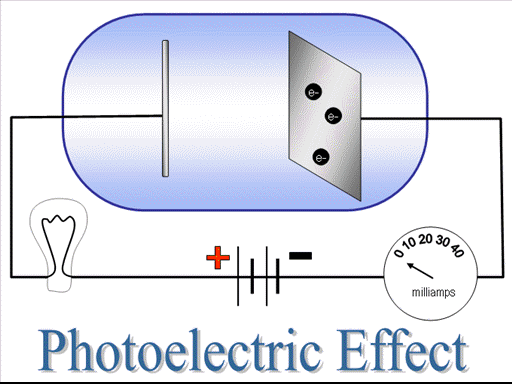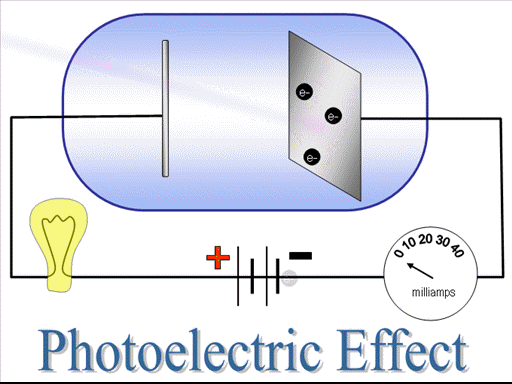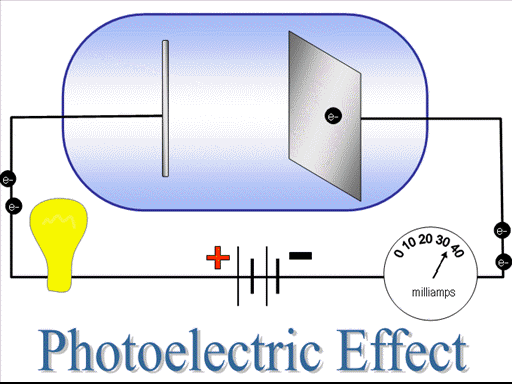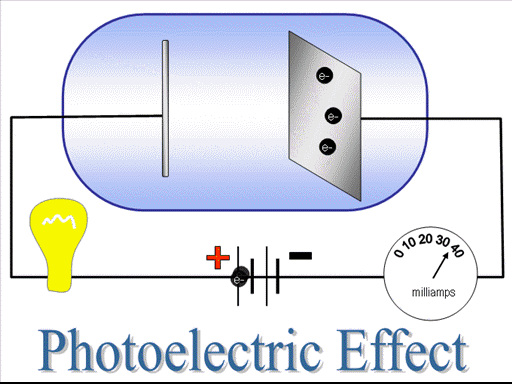
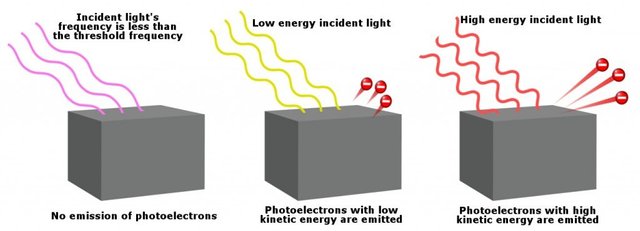
Today i am discussing the reverse of above process i.e by exposing radiations { γ-rays, X-rays or UV-rays} on the metal surface electrons also can be obtained. This phenomena is called photo-electric effect. This phenomena helps us to understand the particle nature of light. The phenomena was successfully explained by Albert Einstein on the basis of quantum theory of light and won a nobel prize in 1921.
Credit
The electrons emitted out in this process are also known as photo-electrons, the kinetic energy of the photo-electrons depend upon the frequency of the radiations. The number of electrons emitted out in this process depend upon intensity of the radiations.
Important features:
Threshold Frequency:
It is not possible to do photo-electric effect with ordinary white light, infrared or radiowaves. Why? Because the photons of these radiations do not have sufficient energy. Only few regions of radiations γ-rays, X-rays or UV-rays can do photoelectric effect. Energy is required to dislodge a metallic electron from its bound state. When a photon incident on a metal surface it must have that energy inorder to supply it to electron, to make it free. The frequency of that photon is called threshold frequency. The kinetic energy of the photo-electrons depend upon frequency of radiations. The kinetic energy of the photo-electrons decreases as the frequency of the radiation decreases and it becomes zero at threshold frequency.
Credit
Work Function:
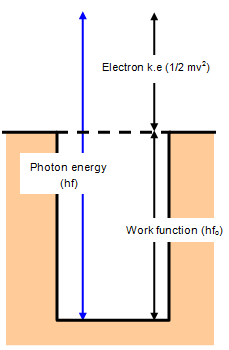
The energy required to dislodge an electron from its bound state and to make it free is called work function. If a photon incident on a metallic surface and its energy is equal to work function, it just make the electron free, but in the case photon has energy greater than work function, its additional energy after setting the electron free will be converted into the kinetic energy of the photo-electron. It is the property of the metallic surface, different metallic surfaces have different work functions.
credit
Stopping potential:
To test the kinetic energy of the photoelectrons, they are exposed to a negative potential. Negative potential repel the photoelectron, if electrons still manage to pass through the circuit this shows that they have sufficient kinetic energy. On gradually increasing the negative potential the time will come when no photo-electron will pass through the circuit, despite of the fact that photo-electric effect is going on. That negative potential where electric current becomes zero is known as stopping potential. The stopping potential depends upon the frequency of the radiations thus depends upon the frequency of the radiations.
Intensity of radiations:
credit
The number of photons passing perpendicular to unit area per second is called intensity of radiations. Increasing the intensity of radiations have nothing to do with energy of the radiations. Increasing the intensity means increasing the number of photons. If one wants to increase the energy, it is linked with the frequency of the radiations. For example the energy of ultravoilet photons is less than energy of x-rays photons because frequency of ultravoilet photons is less than frequency of x-rays photons. Similarly the energy of x-rays photons is less than the energy γ-rays because the frequency of x-rays photons is less than the frequency of γ-rays photons.
Saturation Current:
The electric current obtained in photo-electric effect depends upon intensity of radiations. The larger intensity of the radiations give us larger current and vice versa. The maximum electric current obtained at a particular intensity of radiations is known as saturation current. In order to increase the electric current one have to increase the intensity of the radiations.
Experimetal Results:
Result No.1:
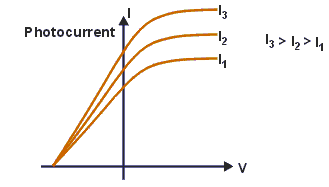
On changing the intensity of the radiations and keeping frequency constant, saturation current will change but stoppong potential will remains constant.
In the graph below, higher intensity is giving more photo-current. Currents are different but all these three curves meeting at one point on negative x-axis which is representing stopping potential.
Credit
Result No.2:
On changing the frequency of the radiations and keeping intensity constant, stopping potential will change but saturation current will remain constant.
In the graph below, higher frequency is giving more stopping potential. But as intensity is not changing , saturation current is same at all frequencies.
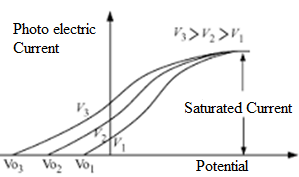
Credit
Result No.3:
The stopping potential is directly proportional to the frequency of the radiations.
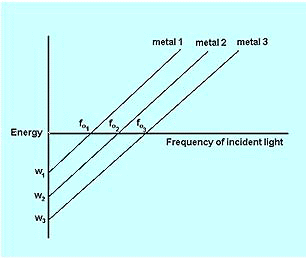
Credit
The place where straight line between frequency and potential cutting the x-axis represents the threshold frequency. Different metals corresponds to different threshold frequencies due to their different work functions.
Einstein's explanation:

Credit
Albert Einstein provided a successful explanation of the photo-electric effect on the basis of quantum theory. He proposed that an electron either absorbs one whole photon or it absorb none. The chance that an electron may absorb more than one photon is negligible because the number of photons is much lower than the electrons, after absorbing a photon, an electron either leaves the surface of metal or dissipate its energy within the metal surface in such a short interval of time that it has almost no chance to absorb a second photon. An increase in the intensity of light source simply increases the number of photons, but the energy of photons remains unchanged. However, the increase in frequency of the light increases the energy of the photons and hence the energy of the electrons too.
Thus according to Einstein's quantum theory, an electron absorbs a photon of frequency to acquire an energy equal to the energy hf of the photon. The electron may loose some of its energy before leaving the metal surface and is ejected with the kinetic energy less than hf. Or, it may loose all of its energy within the metal surface and does not escaspe at all from the metal. This is true even if the photon absorption occur at the metal surface because there exist a force of attraction which holds the electrons within the metals.
According to Einstein a single photon carries an energy hf into metal, where it is absorbed by a single electron. Part of this energy is used in ejecting an electron from trh metal surface. The excess energy becomes the electron's kinetic energy.
Energy of incident photon = work function + Kinetic energy
hf = hf0+ eV0
eVo = hf - hf0
f =frequency of incident photon, f0 = threshold frequency & V0 = stopping potential
[Einstein's photo-electric equation]
The failure of classical theory:
[From classical theory, there should be no threshold frequency because at a given time,(https://www.miniphysics.com/failure-of-classical-wave-theory.html) electrons might absorb enough energy from the incident light to escape from the metal surface at any applied frequency. The classical theory was predicting a time delay for low intensity radiations, but actually it was not present.
The velocity of photoelectrons should depend upon the amplitude of the wave incident on the metal and therefore upon the intensity rather than the frequency.
Photo Cell and their uses:
The photocell or photo tube consist of a evacuated glass tube fitted with an anode plate and a concave metallic cathode of an appropriate surface. The material of the cathode can be chosen to respond to the frequency range over which the photocell operates. The response can be made proportional to the intensity of the light source. A photocell can be used in any situation where beam of light falling on a cell is to be interrupted or broken.
For example:
- To count vehicles passing a road or items running on a conveyer belt.
- To open doors automatically in a building
- To operate burglar alarms
credit
Photo-conductive cell
Besides photo-emission cells there are also photo conductive cells in which an internal photo-electric effect may liberate free charge carriers in a material that is otherwise an insulator, and thereby increase its electrical conductivity by as much as 10000 times when it is illuminated by a light source. Such materials are called photoconductors. A current will flow if the photo-conducting material is in a circuit with a source of electro-motive force. Photo-conductive cells may be used for the following purpose:
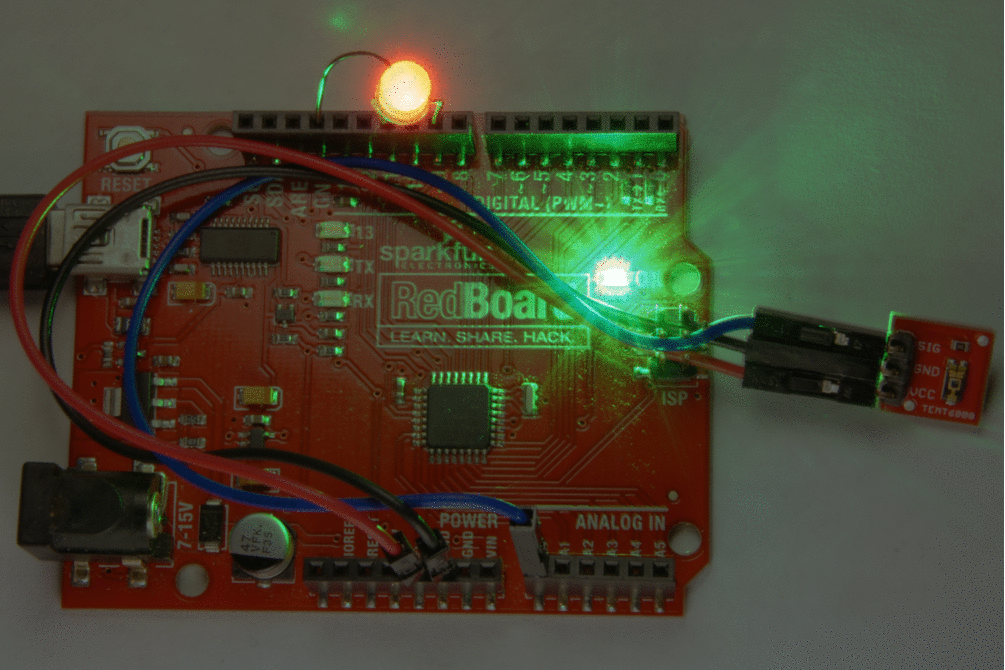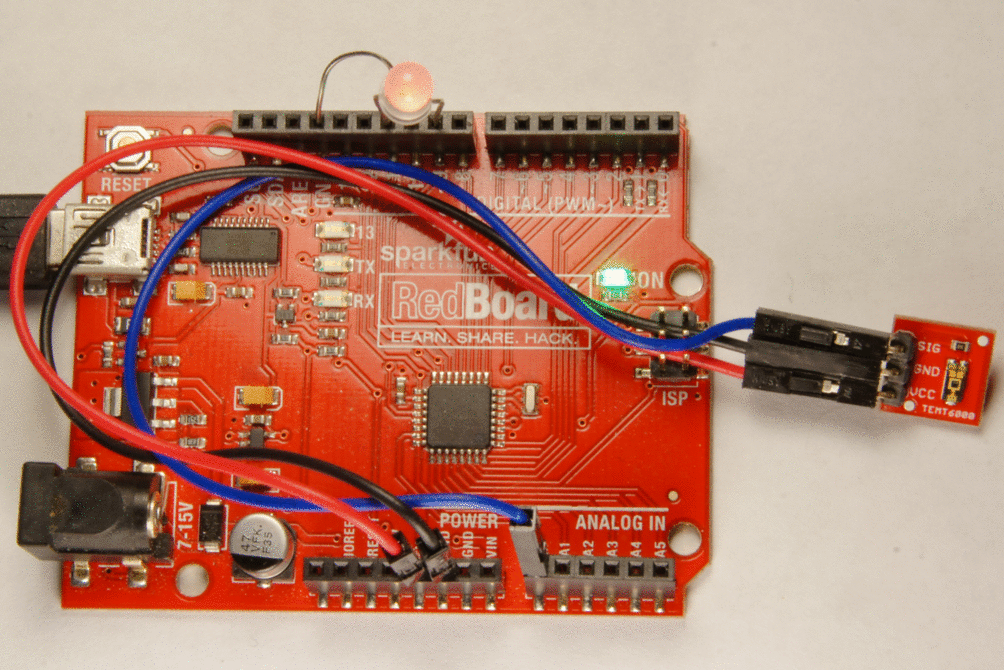
- Detection and measurment of infra-red radiations where the wavelengths is in the order of 10-6
- As relays for switching on artificial lighting such as street lights.
credit
Photo-voltaic cell:
There is yet another type of cell known as photo-voltaic cell which may consist of a sandwich of copper, copper oxide and a thi film(layer) of translucent gold. An e.m.f capable of giving a current of 1mA can be generated when the film is illuminated. Such cells need no source of e.m.f and are frequently used as exposure meters in photography. The exposure meters are required to set the aperture( opening before the camera lens) of the camera compatible to the day light intensity.
Besides the above mentioned uses, the most important use of photocells is of modern everyday life is the production of pictures in television cameras and the sound tracks of motion pictures. In one type of photocell of much usage, the cathode is coated with cecesium, a light sensitive material that emits electrons when illuminated. The electrons collect at a second electrode the anode, the current being proportional to the illumination. The sound information is stored on the film in the spots of varying widths.
credit
When such films run between the light sources the photocell, variations in light intensity reaching the cell cause pulsations in the current that, after being amplified, activates a loudspeaker and reproduce sound.
This is a very nice and complete post, covering all the aspects of the photo-electric effects. Thanks for putting it up.
PS: This Einstein picture is hard for the eyes...
Thankyou! I am glad that you liked it. By the way very true about Einstein picture. Ha ha ha
you just got a new follower im a new student in elctro mecanique and that will help me thanks
@originalworks
The @OriginalWorks bot has determined this post by @shehzad to be original material and upvoted it!
To call @OriginalWorks, simply reply to any post with @originalworks or !originalworks in your message!
Excelente post.
Thankyou!
THANK FOR VOTE MY PHOTO