Improving the Chemical and Physical Characteristics of Metals through Electroplating
"Improving the Chemical and Physical Characteristics of Metals through Electroplating"

When I was still a Chemical Engineering Technology student, I had my on-the-job training at Timex Philippines, Inc., one of the largest electroplating industries in the world, known for manufacturing high-quality watches. I was assigned at the Chemical Laboratory but was also introduced to the Production line to Learn the Overview of the Electroplating Process.
Overview
Electroplating is a process used to coat metals with another thin layer of metal by the aid of electric current, to improve the metal's appearance and anti-corrosion property for its longevity. Electroplating Industries such as automobile, airplanes, electronics and jewelry, use this process to lessen the cost of the raw materials without compromising the overall product and its quality.
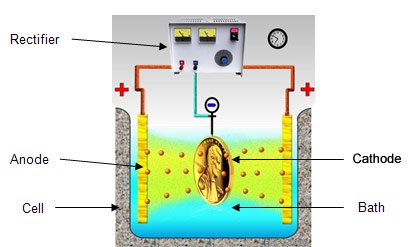
The overall electroplating process happens inside an electroplating cell, consists of a positively charged metal (anode) you are attempting to deposit and a negatively charged metal (cathode) as the substrate you want to electroplate and dipping it into a solution bath that contains metal salt (electrolytes). This requires a rectifier or battery or any power supply for the electrons to transfer from the anode to the cathode .
How Electroplating is Done
Choosing the Right Electrolytes. Determining the right electrolyte is important for the quality of plating, you should consider what type of chemical reactions you want to happen. For example, if you want to copper plate a steel, you need an electrolyte made from a solution of a copper salt.
Determine the Mechanical Finishing the Material Needed. You need to determine if mechanical finishing techniques are required to prepare the surface of the base material before beginning to plate another metal onto it. This is necessary because electroplating does not remove surface inconsistencies.
Cleaning of the Electrodes. This is necessary to ensure that the electrodes are free of contaminants and impurities, oils and greases, as it will hinder the bonding capacity that could lead to unsatisfactory electroplating results. Contamination prevents deposition and lack of adhesion. Generally, there are three steps: cleaning, treatment and rinsing.

Setting-up the Electroplating Station. This is important in every production to prevent bottle-neck operations and other delays. Also to avoid other unnecessary rejected product results. To set-up, you need to connect the negative terminal of the rectifier to the substrate (A rectifier is built to supply current, depends on the production needed but with a very low voltage). Then, place the positive terminal directly to the anode in the plating solution.

Electroplating. As mentioned above, electroplating happens inside an electroplating cell, consists of a tank full of electrolytes and placing bars of the metals (anode and cathode) to deposit into the material to be plated.
When you turn on the electrical current, the resulting electrical field causes movement of metal ions, the anode oxidizes and forms positively charged ions, which are then deposited at the cathode. It will take time for atoms to form on the surface of the negatively charged metal (or cathode). The duration of electroplating process will depend on the intensity of electric current used and the concentration of the electrolyte. Increasing either of these will affect the speed at which ions and electrons move through the circuit thus speeding the plating process.
Final Cleaning and Rinsing. After achieving satisfactory deposition results, post-treatment like cleaning and rinsing is often required to prevent tarnishing of the finished product.
Image Sources: 1, 2, 3, 4, 5, 6
References:
https://chem.libretexts.org/Core/Analytical_Chemistry/Electrochemistry/Electrolytic_Cells/Electroplating
http://www.explainthatstuff.com/electroplating.html
http://www.selectiveplatinginc.com/Electroplating-How-To.html
https://www.thomasnet.com/articles/custom-manufacturing-fabricating/plating-how-to
This post was resteemed by @steemitrobot!
Good Luck!
The @steemitrobot users are a small but growing community.
Check out the other resteemed posts in steemitrobot's feed.
Some of them are truly great. Please upvote this comment for helping me grow.
Thank you @steemitrobot. Here have my upvote. :)
As a follower of @followforupvotes this post has been randomly selected and upvoted! Enjoy your upvote and have a great day!
Thank you @followforupvotes. I appreciate it!
Science chick! Always a good day when steemit has more women posting intelligent stuff! That's awesome. Thank you, I enjoyed reading you.
Thank you. Will be posting for more, I hope you keep updated :)
For sure!
Interesting post shaira!
Thanks @jonelq! Pagpost sa imo ojt haha