Evolution of the Atomic Model: Part 2
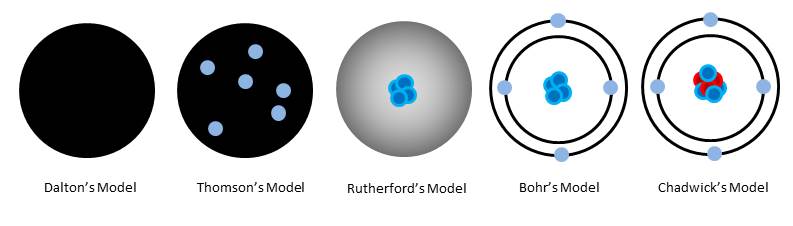
In Evolution of the Atomic Model: Part 1 we learned how the idea came about that matter was made up of discrete particles called atoms. But those discoveries still told us nothing about the structure of atoms themselves. In Dalton’s model, atoms were simply solid, indivisible particles of whichever element we were studying. There was still so much more to discover.
Plum Pudding
In 1897, J.J. Thomson was studying cathode rays, strange rays which were emitted when electricity was passed through empty glass tubes.
It occurred to him that the rays were actually charged particles which were smaller than atoms. These became known as electrons. They have a negative charge and almost no mass. It also occurred to him that these particles actually formed part of atoms. He imagined that electrons in atoms were arranged like plums in a plum pudding – negative electrons embedded in the positive matter that made up the atom.
Alpha Particles
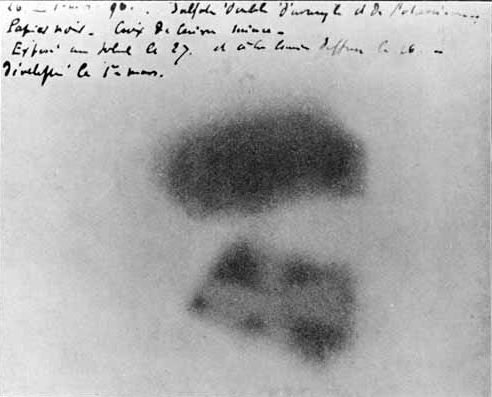
A year before Thomson’s discovery, Henri Becquerel was doing experiments with minerals which glowed in the dark if first exposed to strong sunlight for a while. But one cloudy day, he packed up his minerals and photographic plates (and interestingly, a metal Maltese cross) as it was pointless without the sun, and put everything away in a drawer. Several days later when he took everything back out of the drawer he noticed that his photographic plates had mysteriously developed an image of the Maltese cross. He realised that the only possible explanation was that the minerals were giving off some kind of radiation of their own, and that the cross was blocking some of it, resulting in the image.
We now know that this kind of radiation is a stream of positively charged alpha particles.
Rutherford
This interested Ernest Rutherford, and in 1911 he aimed streams of alpha particles at gold foil. Most of the particles went straight through the foil. But occasionally, a particle would bounce off the foil.
So it became clear to Rutherford that atoms were mostly empty space, but with something small and dense inside. He also deduced that it must be positively charged in order to deflect the positively charged alpha particles, since like charges repel each other just as like poles of a magnet do.
These particles became known as protons. Rutherford’s model of the atom was a tiny nucleus of positive protons orbited by negative electrons, to balance the charge.
Energy levels
In 1913, Niels Bohr was trying to work out why the negative electron didn’t just collapse into the nucleus due to the attraction of the opposite charges. Electrical discharge experiments had shown that very specific frequencies of light were being given off. Bohr’s hypothesis was that electrons settle into fixed orbits, or energy levels, around the nucleus. Specific frequencies of light were given off because an electron lost a specific amount of energy when it moved from one discrete orbit to another.
Neutrons
And so in 1930, two German physicists – Becker and Bothe - were firing alpha particles at beryllium, which caused a completely different type of particle to be emitted – this time with no charge. Then in 1932 James Chadwick discovered that these particles had the same mass as protons. Due to their lack of charge, he named them neutrons.
So, more than a century after Dalton’s model of the atom, chemists finally had a complete model of the atom which allowed them to understand the behaviour of the elements.
There is a lot more going on inside an atom than is depicted in Chadwick’s model, and there are a great many different types of subatomic particles – but that goes beyond the scope of chemistry – best to leave that to the particle physicists!


Well done! This post has received a 50.00 % upvote from @litasio thanks to: @revisechemistry. Whoop!
Promotion: Mining cryptocurrency with Hard Drives! Storagemining.io