The Curious Quantum World: Part 3

1924 a PhD thesis by French physicist and aristocrat Louis de Broglie postulated that the particles of matter can also have wave properties.
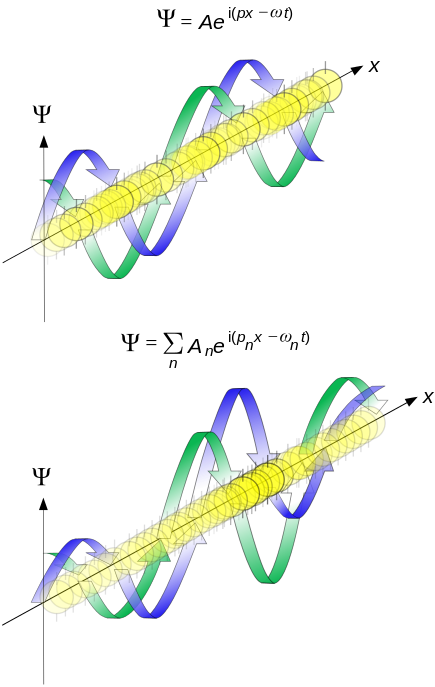
This image represents a propagation of de Broglie waves in 1d. This new view of matter gives a more definite picture of Bohr’s orbits: They represent stationary wave patterns for an electron is in an atom. The old 19th century distinction; matter is made of particles, light is a wave, completely broke down.
In the quantum world, light and matter both have wave and particle characteristics.
Between 1900 and the mid-1920s, physicists extended quantum theory to a wider and wider range of phenomena. Things that were once thought to be smooth and continuous turned out to be discrete and discontinuous. Things that were once thought to be discrete turned out to have continuous wave properties.
All sorts of quantum phenomena were related to Planks Constant h.

Because h is so very tiny, quantum phenomena occur in the microscopic realm. Despite these successes, not until 1925–1926 did a systematic, mathematical quantum theory emerge.
Austrian physicist Erwin Schrödinger and German physicist Werner Heisenberg developed what looked like completely different quantum theories.
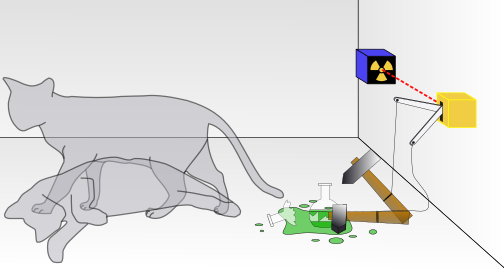
They turned out to be different mathematical forms of the same mechanics. Quantum mechanics is the most successful theory of physics ever created, explaining the nature of light, the structure of matter, and the behavior of particles.
The main features of quantum mechanics are wave-particle duality, indeterminism, and entanglement.
1. wave-particle duality 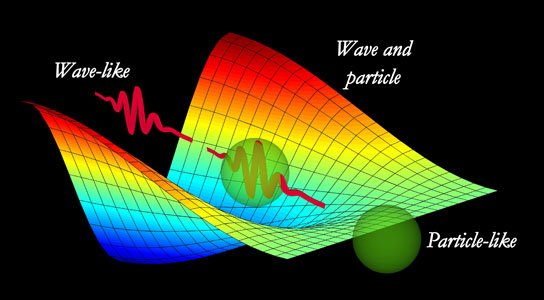

2. indeterminism 

3. entanglement 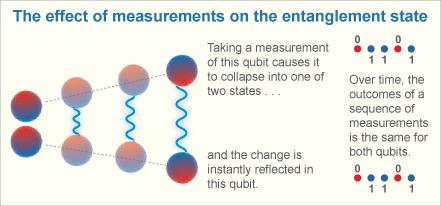

These 3 basic features take us to some remarkable places. Because Planck’s constant (h) is so small, we sometimes say that quantum physics is only important in the microscopic realm. But of course this is not really true. Many large-scale facts about the world that are determined by quantum physics.
END PART 3
PART 1, PART 2, PART 3, PART 4, PART 5
Image Credits (in order):
http://www.science4all.org/wp-content/uploads/2013/01/atom711.jpghttps://upload.wikimedia.org/wikipedia/commons/thumb/2/21/Propagation_of_a_de_broglie_wave.svg/435px-Propagation_of_a_de_broglie_wave.svg.pnghttp://vzn1.files.wordpress.com/2013/02/quantum_corral_nise.jpghttp://www.azquotes.com/picture-quotes/quote-all-matter-originates-and-exists-only-by-virtue-of-a-force-we-must-assume-behind-this-max-planck-23-29-18.jpghttps://upload.wikimedia.org/wikipedia/commons/thumb/9/91/Schrodingers_cat.svg/504px-Schrodingers_cat.svg.pnghttp://scitechdaily.com/images/Mystery-of-Wave-Particle-Duality.jpghttps://sites.google.com/site/phil1300e/_/rsrc/1233028564629/Home/moral-obligation/free-will/543682312_dd6c28eed0.jpghttp://www.research.att.com/export/sites/att_labs/library/image_gallery/articles/2011_Jan-Mar/201101_photon_measuring_qubits.png
I enjoy your posts. Another small lesson. Thanks!
Following too :)
Thanks for checking them out. I try to keep things fun and accessible.
Does light (both a wave & particle) exist at the quantum level? I mean, I assume it does, but how is it different? Do we observe quantum phenomena with light? This is probably a dumb question, but once our eyes see it, the information has already been translated up onto larger scales. Are quantum distances, for example, shorter than a wavelength of light? Or does something like that matter?
Light (photons) exist as probability waves in the electro-magnetic field. Measurements of these fields reveal standing wave patterns where the "particle" is attributed to the position of highest amplitude in the given measurement.
This quantum measurement or observation destroys the original probability wave because it interacts with it. In the macroscopic world such "measurements" always are a part of information from the quantum world transitioning into the macroscopic world.
The plank length 1.616199(97)×10^−35 metres which can be considered the smallest distance in quantum physics is derived from the relationship between the minimum wavelength of light and the minimum energy per quantum. Wavelengths of light smaller than this distance would have undefined properties meaning they could have any (including infinite) energy, density, temperature, size or other properties. Such distance can be considered to be irrational.
Wow. I'm surprised there are answers to my questions. And impressed that you know what they are!
To add one little something, you can get the wave nature of light by studying interferences or anything related to waves, as @pjheinz is mentioning. You can get the particle nature of it in photon collisions at particle colliders or particle decays into pairs of photons.