How and What Bacteria ‘Breathe’
First, Congratulations to Dianne Newman on being named a MacArthur Fellow
When I was in graduate school, I remember discussing with Dianne the ways that bacteria use sulfur and even arsenic compounds to get their energy. She was into the microbiology, and I was interested in the impact of bacteria on the mobility of arsenic and other contaminants in soils and water. Several years after that I saw her present some results at one of the big AGU meetings. She was a nice person and a brilliant scientist. I’m pleased to know that she’s now a professor at my undergraduate alma mater, Caltech.
Dianne talks about bacteria for this story in the Los Angeles Times:
“They are so much more interesting than we are,” Newman said. “We basically have two ways of making energy. These guys have evolved ways to grow under every conceivable habitable condition.”
Seeing that article gave me the idea to introduce the different ways that bacteria derive their energy in different environments. I studied this for my PhD, concentrating on the effects that the chemical transformations have on either mobilizing or immobilizing trace metals and other chemicals in soils and water. Others study this to understand basic microbiology, and still others to understand the fate of carbon and other minerals buried in sediments on the ocean floor or at the bottoms of lakes.
Different roles for different microbes
Here’s an explanation of the different terms applied to microbes in general, including algae, bacteria, and the even older archaea, to classify them according to how they derive their energy for growth. Here ‘fixing carbon’ means converting inorganic carbon (gaseous CO2 or its dissolved forms) into organic carbon.
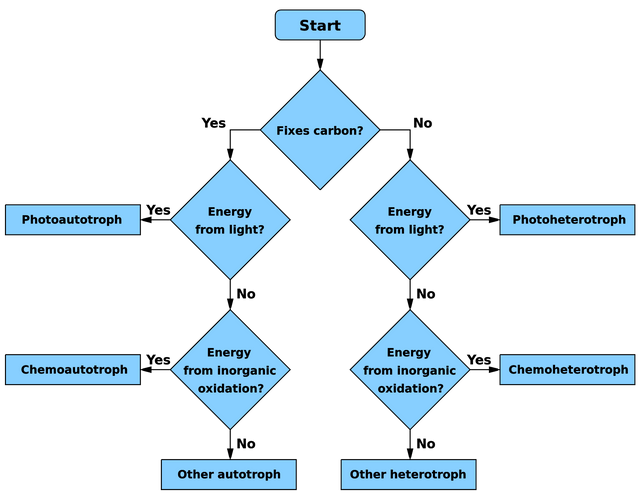
Bacteria ‘breathe’ a great variety of different substances
It’s not exactly breathing, but respiration. We often use the terms to mean the same thing, particularly in a medical context. Humans breathe in oxygen so that we can carry on respiration, which in our case means reacting the oxygen with organic carbon from our food. Our respiration produces energy, water, and carbon dioxide (CO2). Many strains of bacteria can carry out respiration using other chemicals, in places where oxygen isn’t available.
Terminal Electron Acceptors
All forms of respiration transfer electrons from a source, commonly some organic carbon-based source, to some other type of molecule or element. The ultimate destination for those electrons is called the ‘Terminal Electron Acceptor’. For humans, it’s oxygen. Oxygen atoms have a high affinity for electrons, and that’s why aerobic respiration yields more energy than the other respiratory pathways below. More precisely, the electrons are in a considerably lower energy state when they’re around an oxygen atom then when they’re around a carbon atom. That’s why moving electrons from organic matter to oxygen yields an appreciable profit in terms of energy for us, and for all creatures that respire using oxygen.
Respiration using the other common electron acceptors yields less energy than aerobic respiration. Organisms that carry out fermentation --which is a more general term than merely for the production of ethyl alcohol-- actually use other organic molecules as terminal electron acceptors.
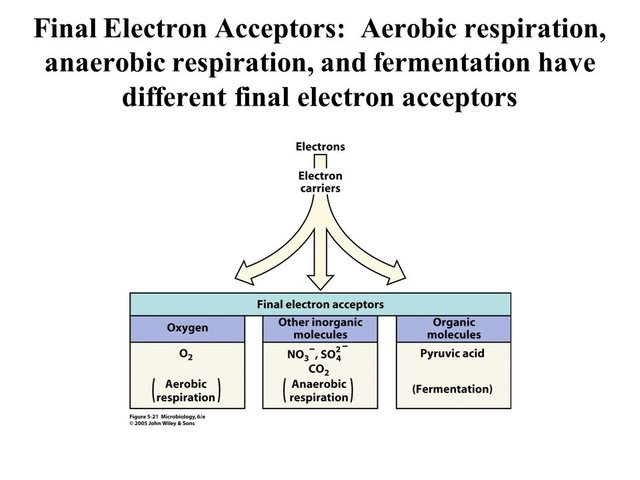
This image is from Gary Andersen’s presentation about microbial nutrition.
Here’s a figure showing the typical cascade of electron acceptors in marine sediments. It’s pretty much the same for freshwater sediments, too.
The source for the above image can be found here.
Oxidizing one thing means reducing something else. So, these reactions are in general called oxidation-reduction, or Redox, reactions.
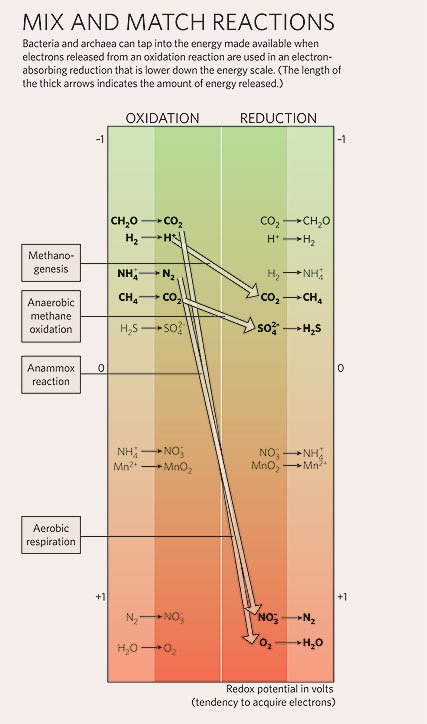
From Microbiology: Batteries not Included. What can’t bacteria do? by Nick Lane (Nature 2006).
Organisms that use the other electron acceptors can only dominate in environments without oxygen, because with oxygen present they would lose out to other organism that gain more energy and therefore grow faster using oxygen. Similarly, nitrate-reducing organisms get more energy than iron-reducing organisms. In general, the faster-growing organism who use the more energetically profitable electron acceptors are found at shallower depths than the slower-growing organisms using use the less profitable electron acceptors.
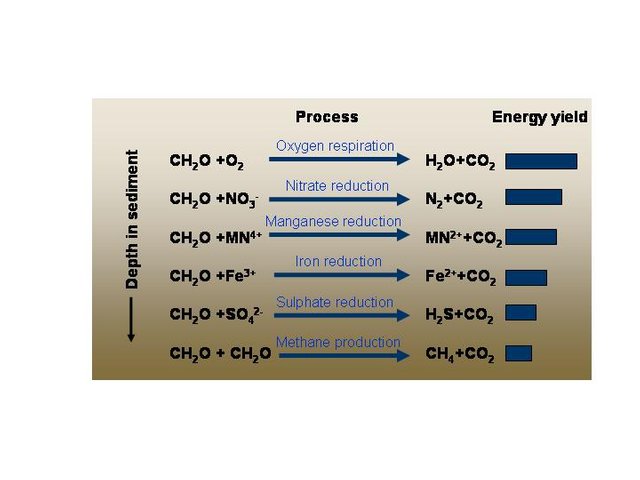
This figure shows the relative energy yield of using different terminal electron acceptors for respiration. “CH2O” is used as a shorthand to represent organic matter in general.
Complex cycles of chemical reactions between the different electron acceptors, recycle energy and maintain gradients in the concentrations of these chemicals in sediments.
Gemma Reguera explains in more detail in his blog post, Living Wires of the Ocean Floor. Here’s one of his nice figures showing the typical pattern in relative concentrations of the different electron acceptors with depth in sediments.
These cycles of energy and elements are central to what’s come to be called biogeochemistry or biogeochemical cycles, as I featured in my previous post about Parallels between the Contrasting Approaches within Ecology and Economics, respectively.
S. Lan Smith
Kamakura, Japan
September 22, 2016
Thanks to everyone who produced and made freely available the images used in this post.
This is great. I've just been reading the first five issues of OSC's 'Ultimate Iron Man,' with the bacterial armor that eats metal. http://www.hatrack.com/misc/ultimateironman/

And tomorrow I'm heading off to this conference on astrobiology, which will not focus on microbes.
http://kcs098.wixsite.com/socia