How does increasing the temperature affect the rate of reaction?
Investigating the Effect of Temperature on Reaction Rate
Aim:
I want to investigate how temperature effects the reaction rate.
Equipment:
Conical Flask
Beaker
Measuring Cylinder
Stopwatch
Waterbath
Paper with a black cross "X"
Thermometer
0.1mol/L Sodium Thiosulfate
2.0mol/L Hydrochloric Acid
Method:
- Put the piece of paper with the X on the bench and put the conical flask on it.

- Measure 50mL of Sodium Thiosulfate and put it into a beaker.
- Record the temperature of the Sodium Thiosulfate.

- Measure 5mL of HCl and put it into the conical flask.
- Pour the Sodium Thiosulfate into the conical flask, start the timer and swirl the flask.
- Stop timing when the you can't see the cross on the paper.

- Wash out the flask thoroughly.
- Repeat the experiment with the Sodium Thiosulfate at 30°C .
- Repeat the experiment with the Sodium Thiosulfate at 40°C .
- Repeat the experiment with the Sodium Thiosulfate at 50°C .
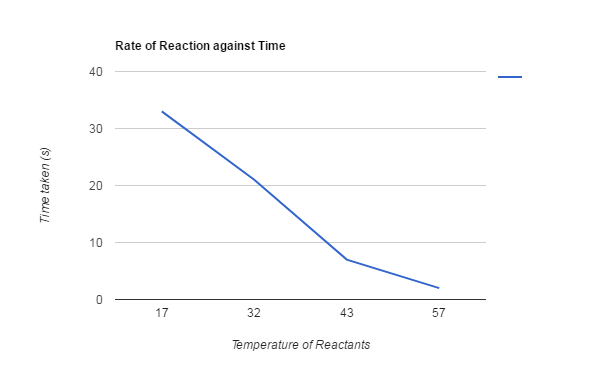
Results:
17°C took 33sec for the X to disappear.
32°C took 21sec for the X to disappear.
43°C took 7sec for the X to disappear.
57°C took 2sec for the X to disappear.
Conclusion:
A chemical reaction is when two reactant collide.
They must collide with enough force and in the correct orientation.
As I increased the temperature, the reactants gained more kinetic energy and moved faster.
The faster they move, the higher the chance of a successful collision.
This means the rate of reaction will increase.
So what happens to say the reaction of a biological enzyme such as human DNA Polymerase delta, if I were to raise its reaction temperature to say 57 C and measure its functional catalytic rate of copying DNA? Does it too go faster?
What a great question. My best answer would be that as DNA polymerase is a protein and proteins get denatured above 45 C, then the reaction would not work. Therefore no rate of reaction. I will be doing a basic catalyst experiment soon. I will try to do a separate experiment that also looks at temperature with a biological catalyst.
You are correct, though if the polymerase we're to remain folded, as with other reactions it's rate of catalysis would speed up.
In the polymerase chain reaction use for amplifying DNA in molecular biology, special thermostable DNA polymerases are used, from organisms that thrive in extremely high temperatures, these polymerases do not unfold as easily, some having stability up to 100 C.
As I read your response, I can't help but think about what temperatures biological proteins can survive? In particular, could a "hitchhiker" survive the hot temperature of a meteorite entering our atmosphere? I am sure this question has been tested somewhere/sometime.