Tears Flow Because of Capillary Action

Ever wondered how it was possible for tears to flow down your eyes. Some times you wished there was no such thing. Well today you will know what made it possible. How tears flow
Tears flow because we cry. We cry whenever we are emotionally hurt, whenever we are extremely happy for some. Tears flow through the lachrymal ducts present behind the eyelids because of what is known as capillary action.
Capillary Action also known as capillarity is defined as the the movement of a liquid through a narrow tube/opening (often against gravity) due to the intermolecular force of attraction between the liquid and the surface of the tube.
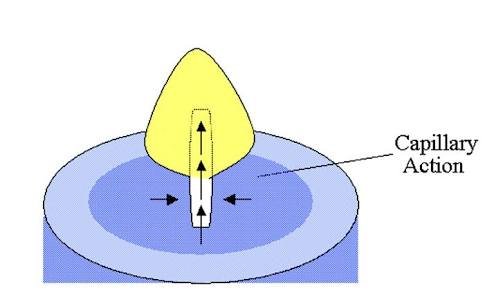
Capillary Action is what we see when we place a narrow tube into a liquid such as water and observe it climbing or moving upward along the narrow tube. It is also what we observe when the edge of a towel is placed in water and water seems to move to other areas of the towel that were not dipped in water
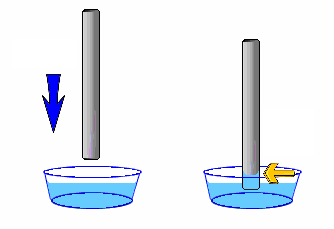
The major intermolecular forces of attraction that exist between liquid and liquid or liquid and solid that give rise to capillary action are cohesion and adhesion.
Cohesion
Cohesion is the force of attraction that exist between molecules of the same liquid. This force of attraction is what keeps liquids as we know them today together and give them their shape. Imagine water molecules separated, more like a grain of water, that is, if any such thing could exist.
Cohesion is the force responsible for keeping rain drops together. The force of cohesion results to surface tension. Surface tension - the force per unit length acting on a liquid - gives water than elastic feel, makes insects, coin, paper clip float on water.
Adhesion
Adhesion is the force of attraction that exist between molecules of dissimilar surfaces. It is what makes water cling or stick to other surfaces such as glass, paper etc.
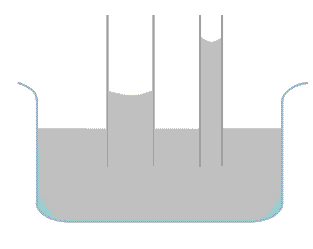
The combination of the adhesive force and cohesive force present in a liquid bring about capillary action. The interaction of liquid molecules with the tube and other liquid molecules alike pull the water upward. The water rises until there is sufficient amount of liquid in the tube for gravitational force to overcome this intermolecular forces.
The more narrow the tube the higher the liquid would rise through the tube. This is because a narrow tube brings the surface for interaction between the molecules closer causing the liquid to rise higher. In the case of lachrymal ducts, they are so narrow that tears flow to the very top.
As in the case of the lachrymal ducts in the eye, the adhesive force and cohesive force between the tears and the duct and the tear molecules respectively bring about the rise of tear from within the eyes through the lachrymal ducts until they get to the surface.
Other places we can see capillary action at work
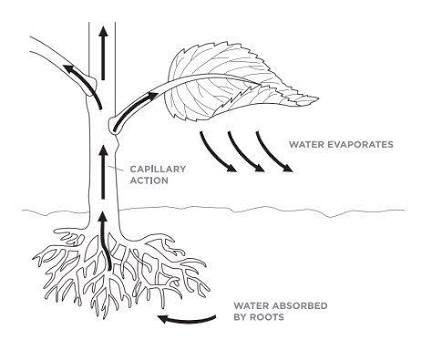
Capillary Action is also at work I plants. Where? Water doesn't magically travel to the leaves from the soil. It is by capillary action that plants are able to transport water from the root through the xylem to areas of need such as the stem, branches and leaves.
Capillary Action is also at work when those candles burn through out the night. The heat from the wick melts the wax and the melted wax moves up through the wick supplying the fuel necessary to keep the fire burning.
Conclusion: Capillary Action works for us indirectly or directly. Whether we notice it or not it is occurring. Hopefully this post has given us a better insight to what really happens. Can you think of any other place capillary action is at work?
Reference:
Image Credit:
- Image 1 from pixabay.com
- Image 2 from www.chim.lu
- Image 3 from Tutorvista.com
- Image 4 from Science World British Columbia
- Image 5 from Wicks Unlimited

Is there a link between capillary action and surface tension? What is the difference BTW capillary action and capillarity?
From my study capillary action and capillarity are the same. The capillary action is a result of both cohesion and adhesion as I read from sources. Where surface tension comes in is through cohesion. Do check the sources from more info
@originalworks
The @OriginalWorks bot has determined this post by @kenadis to be original material and upvoted it!
To call @OriginalWorks, simply reply to any post with @originalworks or !originalworks in your message!
Your Post Has Been Featured on @Resteemable!
Feature any Steemit post using resteemit.com!
How It Works:
1. Take Any Steemit URL
2. Erase
https://3. Type
reGet Featured Instantly – Featured Posts are voted every 2.4hrs
Join the Curation Team Here