Today's Indispensable - Boron
Boron is an element with a semiconductive property between metal and nonmetal, which is indicated by the symbol B in the periodic table, atomic number 5, atomic weight 10,81. It is the first and the lightest member of group 3A in the periodic table. The basic state electron configuration is 1s2 2s2 2p1.

Boron behaves like a non metallic compound in its compounds but purely boron is electrically conductive such as carbon. Crystallized boron is similar in appearance and optical properties and almost as hard as diamond. The pure element of the boron was first discovered in 1808 by the chemist J.L. Gay-Lussac, Baron L.J. Thenard and H. Davy.
Boron is an element commonly found in soil, rocks and water. Boron deposits at high concentrations and economic sizes are found in areas where the arid, volcanic and hydrothermal activity of Turkey and USA. Turkey is in the first place with a share of 73% in the world total boron reserve order with a total reserve of 3.3 billion tons.
Boron and Usage Areas
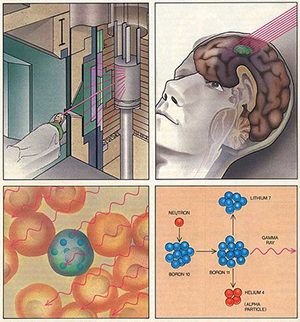
The different properties exhibited by the various metal or nonmetal compounds make it possible to use many of the boron compounds in the industry. Boron is used in the glass and ceramics industry, detergent and cosmetics industry, flame retardants, agriculture, metallurgy, healthcare, cement and energy industry. For example boron is used actively in the health sector, in the treatment of osteoporosis, in allergic diseases, in psychiatry, in bone development and in arthritis, menopause treatment. In addition, Boron Neutron Trapping Therapy (BNCT) has become a new hope in the treatment of cancer, with the boron element serving as a means of destroying cancer cells without damaging healthy cells.
Boron and its compounds are also used as additives in many areas. Borates in petroleum technology function as a polymer cross linker or a retarder in the freezing of cement in drilling pits. Perborates help to degrade and remedy polymer residues. Another useful application of boron compounds to the industry is to prevent the oxidation of aluminum and magnesium metals.
Boron and Energy
Its use in the context of boron energy can be examined under the following headings.
- Hydrogen carrier
- Improvement in lithium ion batteries
- Direct fuel (Elementary boron)
- Increased efficiency in solar panels
- Energy savings in production and reduction in carbon emissions
- MgB2 is a superconductor (with zero loss energy transfer).
Hydrogen Production and Sodium Borohydride as Adsorplicator
There are many methods used for the transition of renewable energy. It is possible to count sun, wind, geothermal resources, hydropower and hydrogen energy among these. Hydrogen is considered one of the most valuable and "green" energy sources in the near future. The biggest problem with using it as an energy source is the risks associated with storage and transport. Extensive researches are underway to obtain a viable solution. Between many hydrides and other hydrogen containing materials as possible hydrogen sources, metal borohydrides have been a unique choice due to their lightness and stability. As hydrogen production and adsorber, sodium borohydride will have an important role in the energy field with the widespread use of hydrogen as fuel. Sodium borohydride reacts with water at room temperature without high pressure and produces hydrogen. This reaction releases a controllable heat and does not produce harmful by-products.
Sodium Borohydride in Fuel Cells

Sodium borohydride can be used as a direct fuel in the fuel cell as well as the hydrogen storage feature. Fuel cells offer many advantages but performance enhancement and cost reduction studies such as membrane development and catalyst reduction are still needed. The method of using borohydride instead of gaseous hydrogen as fuel has many advantages. First of all, the storage problem has been solved and no special tools are required for moving applications, such as dangerous high pressure cylinders or energy consuming appliances that are cooled for liquid hydrogen. Sodium borohydride stands out as a good alternative because of its high volumetric and weighted hydrogen storage capacity at this point. Sodium borohydride can be used directly in fuel cylinders or in two main forms, hydrogen production, when fuel is demanded outside the cylinder.
Lithium Ion Batteries
Lithium ion batteries used in energy storage parts in electronic devices such as mobile phones, portable music players, portable computers are seen as smart technologies of the future. Lithium ion batteries continue to work on properties such as reducing the burning and explosion characteristics of the battery and enhancing chemical and electrochemical stability by adding boron.
Solar Panels

Solar energy is the energy of radiation that is turned on by the fusion process in the solar core (helium conversion of hydrogen gas. Studies on utilizing solar energy has gained momentum, especially since the 1970s, as solar energy systems have emerged as an environmentally clean source of energy, technologically progressive and cheap.
In terms of method, material and technological level, solar energy technologies are examined in two main groups as photovoltaic and thermal solar technologies. Semi conductive materials called photovoltaic cells turn the sunlight directly into electricity. Firstly the heat is obtained from the solar energy and then this heat can be used for electricity generation in the thermal system.
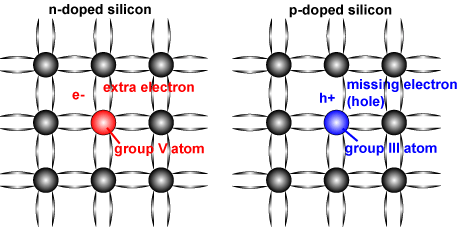
P and N type semiconductor junctions are required to form the photovoltaic cell structure commonly used in solar panels. In order to obtain P type semiconductors, an element (Boron, Gelium, Indium etc.) in group 3A of the pure silicon crystalline periodic table is added at very low concentrations. If boron is added to the silicon crystal, 3 valence electrons and silicon and 4 valence electrons make a covalent bond among themselves, and a valence electron of the silicon atom is idle. The vacant valence electrons of the silicon atom fill the void in the adjacent boron atom. Thus, it is assumed that 1 electron deficiency occurs in the crystal structure of silicon and positively carries it. Such items are also referred to as 'p type' or 'receiver' additives.
References
- N. S. Hosmane, Boron Science- New Technologies and Applications, CRC Press, New York (2012)
- E. G. Camparia, M. Bianchib, L. Tomesanib, Boron as a storage medium for solar energy, Energy Procedia 126 (201709) 541–548.
- http://www.tcichemicals.com/en/us/product/tci-topics/ProductHighlights_20131209.html
- http://hyperphysics.phy-astr.gsu.edu/hbase/Solids/dope.html
- https://en.wikipedia.org/wiki/Boron