Ozone Layer Depletion
We know that the atmosphere is a gas layer surrounding the earth. The atmosphere is made up of the gases needed for living things to survive. These gases are 78% Nitrogen (N), 21% Oxygen (O), 1% other gases. This gas layer has many benefits to the world. Without the atmosphere, the earth would be full of light as soon as the sun was born, and it would be completely dark at the sunset and at the same time bright places would too hot, dark places would too cold and no weather events happen.

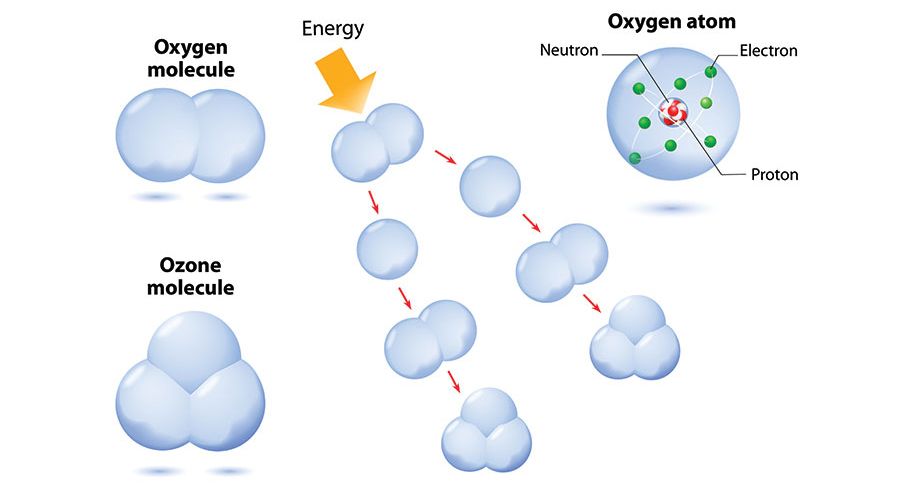
Ozone molecules show different characteristics according to the place they are in the atmosphere. The ozone layer in the stratosphere is beneficial for the living things. In contrast to this, there is a devastating effect of 10% ozone in the atmospheric layer near the surface of the earth. Ozone (O3) is a transparent gas composed of three oxygen atoms. The ozone layer is a layer of ozone gas that is 10 to 50 km above the surface of the earth, in other words, the upper levels of the atmosphere. The main task of this layer is to filter ultraviolet (UV) rays.
Approximately 90% of the total ozone is a layer 20 km thick, scattered in a very cold layer of the upper atmosphere known as the stratosphere, reaching about 25 km in height and 15-45 km above the earth's surface. It's only one in every 100,000 molecules.
Is Ozone Good or Bad?
Good ozone is 90% of all ozone and is also called stratospheric ozone. This ozone layer acts as a natural filter to protect all living things on the earth against the sun's rays. Without the ozone layer, many people would get skin cancer, cataracts, and other diseases. And animals and agricultural crops as well as living organisms at the upper levels of the ocean would suffer from it.
Ozone naturally occurs in the atmosphere and is degraded there. In the atmosphere, ozone, sun rays, nitrogen, hydrogen, and chlorine are decomposed by chemical reaction with various compounds including chlorine. Produced in the atmosphere and consumed ozone is balanced.
The other 10% constitutes bad ozone. It occurs at the ground level with serious air pollution caused by volatile organic mixtures of vehicle exhausts and industrial emissions, as well as human activities resulting from the mixing of nitrogen oxides into the air. On hot days in the summer, volatile organic compounds and nitrogen oxides react with sunlight to form hazardous urban industrial haze called smoke. Bad ozone can cause severe eye, nose and respiratory problems in humans and animals, and can damage forests with agricultural products.
Depletion of The Ozone Layer!
You have absolutely heard that the ozone layer is depleted. As a matter of fact, it means that the ozone molecules do not disappear completely but they decrease rapidly. The result is a thinning of the ozone layer in serious dimensions.
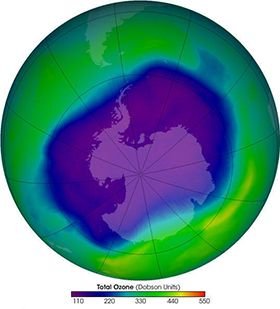
Scientists surprised everyone by explaining the extreme thinning of the ozone layer and the discovery of the hole on the Antarctic continent in 1985. Found that the ozone concentration on Halley Bay (Antarctica) was 40% less than in the 1980s. As a result of careful measurements and intense studies by scientists, it concludes the ozone layer began to be studied in the late 70's.
Substances causing the ozone layer to become thinner;
- Chlorofluorocarbons (CFC)
- Halons
- Methyl Chloroform
- Carbon Tetrachloride
- Hydrobromofluorocarbons (HBFC)
- Hydrochlorofluorocarbons (HCFC)
- Methyl Bromide
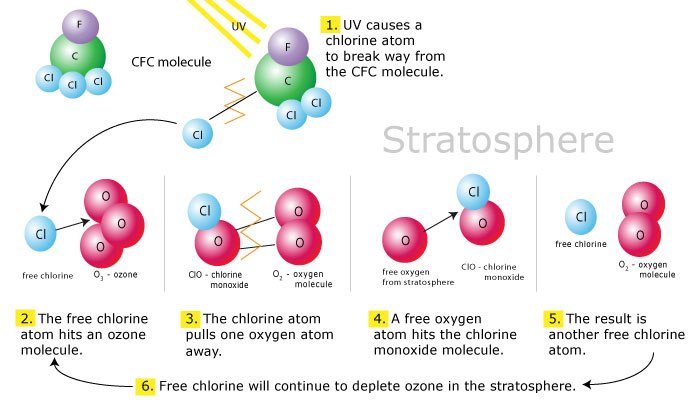
All these chemicals are members of a broad class of chlorine and bromide-containing components known as industrial halocarbons. All these industrial halocarbons are ozone consumers for two reasons. The first is not reactive, they are chemical substances that can be dragged into the stratosphere without deteriorating in the downstroke, and the second is to help the natural reactions that destroy the ozone.
Once these have reached the stratosphere, UV radiation breaks them up by expelling two powerful ozone consumers, consisting of chlorine (CFC etc.) and bromine (halons etc.). Chlorine and bromine both stimulate and accelerate reactions that destroy ozone without being altered and self-destructing. Chlorine atoms have an insatiable appetite against ozone, and a single chlorine atom has the ability to destroy 100,000 ozone molecules.
Can Ozone Layer Depletion Be Stopped?
Scientists say that the ozone layer can slowly regenerate itself if all countries stop producing and using chemical substances that damage the ozone layer in the foreseeable future.
Of course, repair of the ozone layer will not be in a night. Ozone-depleting chemicals will take many years to reach the stratosphere, and they will continue to damage the ozone layer for years, possibly once they reach it, as their depletion will take place over a period of time measured in centuries. The chlorine level in the stratosphere will not begin to decrease until the 21st century, although the production and use of all ozone-depleting chemicals will be discontinued. The ozone layer will estimate to be normal until 2060, that is chlorine level before 1980. When this date is reached, it is thought that the anthropic ozone hole will be permanently destroyed.
As humanity, we must be pioneers in ending this desperate use and consumption madness. We must pay more attention and warnings, thinking that every chemical we use can damage the ozone. For a better life…
Thank you for an enlightening post about the ozone layer. I thought the hole had somewhat resorbed following the widespread ban of Freon gases and such but it looks like the problem is far from being resolved.
Thanks for your interest. To my knowledge, Freon cooler systems have banned under the Kyoto Protocol a long time ago but as you said the problem is far from being resolved for now.
Nice article Kedi, I heard that it had started to repair itself over recent years. I might be wrong....
Did you know that as well as ultra-violet light acting as a catalyst, lightening also produces ozone.
I also liked your final message, people need to sort their priorities out, we are slowly messing this planet up!
Thanks Benjamin. You've got a point. Efforts to repair the ozone layer on Antarctica have begun to yield results recent years. New researches have shown a 20% reduction in ozone depletion damage due to chlorine between 2005 and 2016. Btw, I had read something about lighthening related ozone before.
Nice photos. they deserve an award. It is very nice. great work