Materials of The Future - Smart Polymers
Nanotechnology has the most popular research and development in all basic disciplines. Polymer science and technology has gained a great importance in these researches.

Hermann Staudinger first suggested the existence of polymers that are alternatives to natural materials, whose resources are nowadays decreasing day by day, with the definition of "macromolecules". Polymers contain many factors such as nanoparticle drug delivery systems, sensors and biosensors, biological laboratory devices, fuel cell polymer electrodes, self-organizing polymer films, polymer vessel technology, medicine, veterinary medicine, agriculture. Polymers have the advantages of easy handling, mechanical behavior, flexible construction and low density, which makes the usage area increasingly widespread. Three-dimensional polymers, called hydrogels, do not dissolve when interacting with water, but absorb a large amount of water and become soft. Superporous hydrogels that swell by absorbing water from 10 to 1000 times their original weight in very short durations (about 30 seconds) are being evaluated by researchers especially for the development of new types of biomedical devices and promise a great future in terms of other.

The SEM (Scanning Electron Microscope) image of a hydrogel specimen on the left side gives us striking results about the surface of the polymer. Reminiscent of a sponge, this structure is often used in the field of drug release and biotechnology, as it is biocompatible.
What I mean briefly above is hydrogels, ambient hydrogels, in other words "Smart Hydrogels", which show large and sharp changes to the smallest physical and chemical stimuli from the surroundings. The smartness of any material is to generate responses that perceive and benefit from external stimuli. Hydrogels show changes in swelling behavior, lattice constructions, permeability properties or mechanical strengths against different stimuli (temperature, pH, magnetic field, electrical field, light etc.)
How smart is the "Smart Hydrogel"?
The most study polymer on it is undoubtedly poly N-isopropylacrylamide, shortly PNIPAM. Unlike other materials, the most important feature of this polymer is its shrinkage with increasing temperature. This polymer shrinks when the temperature rises above a certain value. This boundary temperature value is called the lower critical solution temperature (LCTS). Under this temperature, the polymer chains expand and the polymer dissolves in water. The polymer is insoluble above a certain temperature. Another important feature of this polymer is that it is sensitive to heat, that is, its swelling and shrinking reactions against temperature changes are reversible.
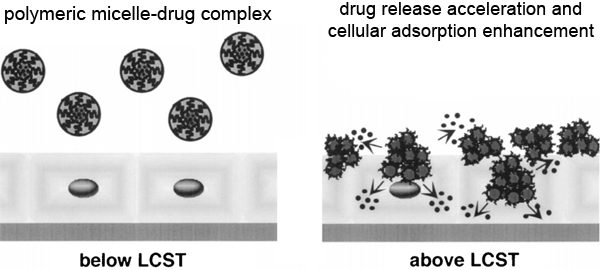
This rapid response allows the jellies to be used as muscles in robots or other mechanical devices or in human prostheses. One of the most important uses of medicine in rapidly developing technology is controlled drug release. What is important here is that the drugs are given to the necessary organs at the desired doses and for a certain period of time. In recent years they are using polymeric structures to release drugs at constant speed.
Smart jellies play an important role in the development of these systems. Since the gel is sensitive to the conditions inside the body, it will be able to maintain the drug level at the appropriate level by changing the release rate. With the use of jellies that are sensitive to temperature or other physical conditions, drug molecules can be trapped in the gel mesh and released into the environment in accordance with the change in temperature. For example, when PNIPAM is used, the gel is dispersed in the gel at 25 ° C. This drug loaded gel is separated from the water jelly with the drug at body temperature and the gel shrinks. In hydrophobic drugs, on the contrary, the gel over LCTS is confined to the structure and the external atmosphere is released at temperatures below the LCTS.
Another important group of smart jellies are also pH sensitive jellies. These are ionic network structures in which swelling or shrinkage behavior is observed depending on the pH.
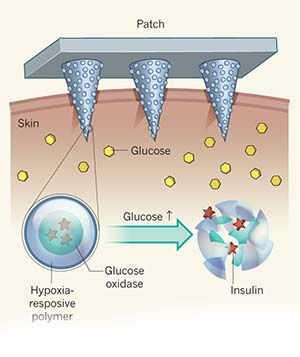
One of the studies on drug release in this regard is to use smart jellies to protect drugs from acidic stomach environment. Smart jellies become permeable by swelling in the intestines (pH> 7) while shrinking at low pH, so passing unaffected by the midline. Thus, release of the drug in its proper condition is ensured. An another application is the release of insulin used in the treatment of diabetes. The gel prepared for this consists of a reservoir containing insulin and poly (methacrylic acid-polyethylene glycol) membrane surrounding it. The glucose oxidase enzyme is trapped in this copolymer membrane. The membrane has a porous structure and openings (molecular gates) are present on it. At high pH values (eg normal body pH 7,4) the gel expands to close the gates. When the level of glucose on the side increases, the enzyme of glucose oxidase, trapped in the zard, reacts with glucose, causing the pH to decrease to 4. At this low pH the gel is shrinked to open the gates and release the insulin.
Is that all features of smart polymers? So, can an alternative method of chemotherapy be found? Nanotechnology continues to study in this area, and the closest method is to use polymers...
Hydrogels sensitive to magnetic fields are hydrogels that swell and shrink due to the magnetic field. It occurs when colloidal magnetic particles are incorporated into cross linked NIPA and PVA hydrogels. When the hydrogel is heated when it enters the magnetic field, it becomes cold when the magnetic field is lifted.
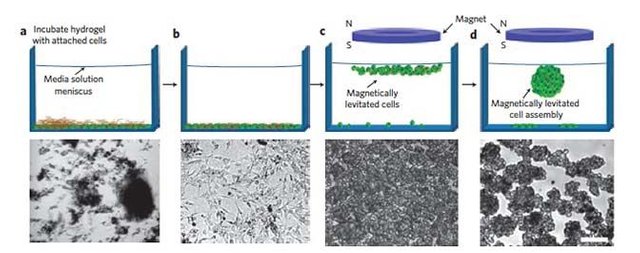
I want to talk about a study. In a study on cancer cells, magnetic susceptible hydrogels were moved to the damaged cell (controlled by a magnet) remotely and warmed by the applied magnetic field. This warming was done at a temperature of 40-45 oC and a decrease in the cancer cells over time was observed. This treatment is called "Hyperthermia Treatment". In the experiment, the applied magnetic field warmed the gel and the bacteria on the gelatin died with the effect of heat, while the remaining cells survived on the other side.
Image Sources: 1, 2, 3, 4, 5, 6
References:
- Paul, D. R., Robeson, L. M., 2008. Polymer nanotechnology: Nanocomposites. Polymer 49 : 3187-3204
- Okay, O., 2010. Polimerlerik Malzemelerin Bugünü ve Yarını Konferans Notları.
- Bayraktar, İ., 2012. Manyetik Hidrojellerin Sentezi, Karakterizasyonu ve Soğurum Özelliklerinin İncelenmesi. Yüsksek Lisans Tezi.
- N. A.Peppas, 1987, Hydogels in Medicine and Pharmacy, CRC Pres, Boca.
img credz: pixabay.com
Nice, you got a 19.0% @trafalgar upgoat, thanks to @kedi
Want a boost? Minnowbooster's got your back!
The @OriginalWorks bot has determined this post by @kedi to be original material and upvoted(1.5%) it!
To call @OriginalWorks, simply reply to any post with @originalworks or !originalworks in your message!
What a funny abbreviations (scientists are known to be bad in abbreviating!)
Do you have more information about the usage of the hydrogels in the cancer treatment? I am very curious about that.
My research contiunes to explore more things about that intriguing subject. I can write one more topic when I finish research. I am also very curious about that.
I am totally looking forward to read this. I will stay tuned for your posts :D
I read your post. I like it very much. Thanks for sharing
Nice post. Didn't know about hydrogels until now.. Thank you