Why Prophet Mohammed Forbid Moslem to Exhale The Hot Water ?
Carbonic Acid
Base is chemical compounds receive the hydrogen ions. For example, bicarbonate ion HCO3-, is a base can receive H+ ions to be carbonic acid (H2CO3). Likewise phosphate (HPO4) is a base because it can form phosphic acid (H2PO4). The function of proteins in body is as bases because some of amino acids build proteins by received of hydrogen ions.
Strong acid is dissociate acid rapidly and mainly release large amounts of H+ ions in solution. The weak acid has less tendency of dissociate ions, therefore it less to releases of H+ . For example H2CO3. A strong base is a fast and strong base with H+, therefore rapidly remove from solution. Examples of hydroxyl ions (OH-), which react quickly to form water (H2O). The weak base is a weakly reacts with H+ ion. For example HCO3-.
Hydrogen ions is an important thing of enzyme system activity in body, because it can affected by hydrogen ion concentration. The concentration of hydrogen ions the body is maintained normally at low level, if compared with other ions, the concentration of hydrogen blood is normally maintained within strict limits of normal value of about 0.00004 mEq/liter. Usually the concentration of hydrogen ion is mentioned with logarithmic scale by using pH unit.
pH = log 1/H+
pH=-log H+
Normality of H+ is 0,00000004 Eq/liter.
Therefore normal pH is :
pH= -log (0,00000004)
pH= 7,4
From above formula, the pH has inversely related of hydrogen ion concentration. Therefore low pH is associated with high concentrations of hydrogen ions and high pH is associated with low hydrogen ion concentrations.
A person is said was acidosis when the pH falls from the normal value and is said to be alkolosis when the pH is above the normal value. The low limit of the pH value at which a person can live several hours is about 6.8 and the upper limit is about 8.0.
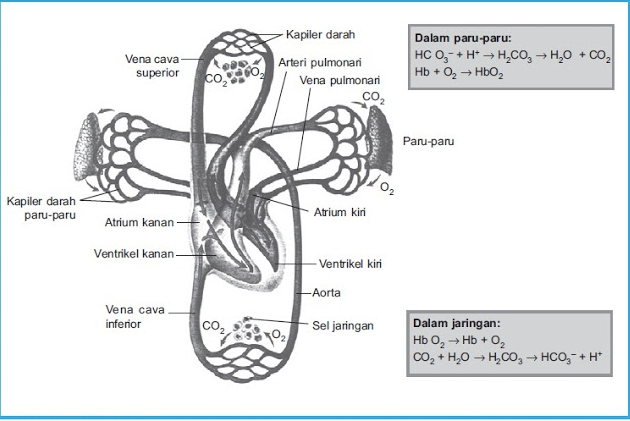
Hot water (H2O) meets the carbon dioxide (CO2) exhaled by mouth, will produce H2CO3 (carbonic acid) compounds.
H2O + CO2 ⇄ H2CO3 H2CO3 ⇄ H+ + HCO3-
The Buffer System of Bicarbonate
The system comprises water solution containing two substances: a weak acid H2CO3 and bicarbonate salt of NaHCO3
H2CO3 is formed by reaction of CO2 and H2O in the body, which is catalyzed of carbonic anhydrase (H2O + CO2 ⇄ H2CO3) enzyme. This reaction is slowly and very produce little amount of H2CO3, unless there is enzyme carbonic anhydrase. This enzyme is especially numerous in the lung alveoli wall and renal tubular of epithelial cells.
H2CO3 ionizes weakly and be small amounts of H+ and HCO3-.
H2CO3 ⇄ H+ + HCO3-
If strong acid such as HCl added to bicarbonat solution, the increase of hydrogen ions is released from the acid.
HCl ⇄ H+ + Cl-
As a result, more H2CO3 formed is causes by increased of CO2 and H2O production. From this reaction it can be observed by hydrogen ions from strong HCl acids react with HCO3- to form weak acids of H2CO3 which forms of H2O and CO3. The second component of this system is bicarbonate salt (NaHCO3), This salt ionizes to form sodium ions of bicarbonate (HCO3-)
NaHCO3 ⇄ Na+ + HCO3-
If the strong base (NaOH) is added to bicarbonate solution
NaOH + H2CO3 ⇄ NaHCO3 + H2O
The Hydroxil (OH-) of NaOh combines with H2CO3 to be additional HCO3-. Thus a weak base of NaHCO3 replace the strong NaOH. At the same time, the concentration of H2CO3 (because it reacts with NaOH), Cause the CO2 join H2O to replace H2CO3.
Therefore, the end result is tendency of decrease CO2 blood level, but this decrease inhibits breathing and lowers CO2 expiratory rate. Increased HCO3- in the blood is compensated by increased HCl-kidney excretion. The end result is the conversion both of strong acids and weak acids and strong bases to weak bases.
Source :
Exchange Mecanism
Follow Me @jamhuery


@jamhuery got you a $0.95 @minnowbooster upgoat, nice! (Image: pixabay.com)
Want a boost? Click here to read more!
This post has received a 0.52 % upvote from @drotto thanks to: @banjo.
Nice article, although I am very surprized by the title :)
thanks @lemouth, i just explain with reaction when someone exhale a hot water,
I was more thinking about the religious reference. Is this forbidden in Islam (sorry, I am very bad in religions)?