The Chemical Science Behind The Fireworks
Potassium Nitrate (KNO3)
Colors of fireworks come from any variety of metal compounds, especially metal salts. The most important component of fireworks is, of course, gunpowder, or 'black powder' as it is also known. This compound was discovered by chance by Chinese chemists, who initially wanted to find a panacea in life, they found that the combination of honey, sulfur and belching (potassium nitrate) suddenly exploded in the fire during warming up.
Sulfur (S)
The combination of sulfur and potassium nitrate is then joined with charcoal in place of honey, sulfur and charcoal serves as a fuel in the reaction, while potassium nitrate is useful as an oxidizing agent. Modern black powder has a weight ratio of potassium nitrate, charcoal and sulfur weight.
Burning of black powder does not occur as a single reaction, so the product can be more complicated. The closest reaction can be written by the equation for this process shown below, with charcoal called the empirical formula:
6 KNO3 + C7H4O + 2 S → K2CO3 + K2SO4 + K2S + 4 CO2 + 2 CO + 2 H2O + 3 N2Variations of the size of pellets from gunpowder and the amount of moisture can be used to significantly increase firework firing time.
Like gunpowder, fireworks contain a 'binder' - which is used to bind components together, and also to reduce the sensitivity of shocks and impacts. Generally these binder compounds comprise an organic compound, ie dextrin, which can then act as a fuel after ignition. An oxidizing agent is also required to produce the oxygen needed to burn the mixture, in which it is usually used as nitrate, chlorate, or perchlorate.
Stars
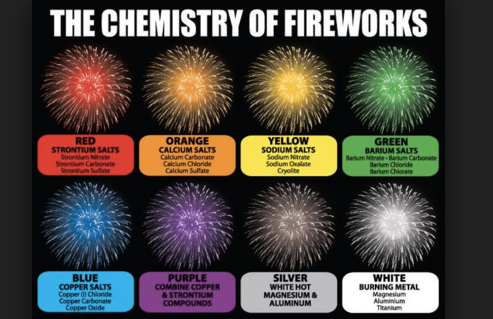
Explosions such as 'stars' contained in fireworks tubes contain metal powders or salts that give the color of fireworks. This material is often coated with gunpowder to aid in ignition. The heat released by the combustion reaction causes the electrons in the metal atoms to excite to a higher energy level. This excitation form is unstable, so the electrons quickly return to their original energy (or ground state), and emit excess energy as light. Different metals will have different energy gaps between the ground state and the excited state, leading to different color emissions. This is exactly the same reason for the flame reaction of metal, that different metals provide different fire tests, which we often use to distinguish between the metals.
The colors emitted by different metals are shown in the graph below.

Follow Me @jamhuery
Fireworks are integral part of any festival. But few people know the science behind it. Good writing!
Mantap
Follow me my friend @jamhuery