Chemical Magic #1 : Electric Generator From KITCHEN SALT
Alternative Energy
Alternative Energy

In this post, i will explain how salt solution can provide DC electrical energy or current electric direction. For formation of energy requires of zinc metal and copper to be smuggled to salt solution. Alternative energy is the energy currently being searched by scientists in exchange for energy with fossils. The scientists very worried about depletion of petroleum and coal energy on earth due excessive utilization. Today many countries are developing alternative energy to use as a source of electricity in an area. Recently many young scientists developed a variety of simple tests to get electrical energy for free, such as power plant with magnets, earth gravity and brine.
The discovery of energy source with economical material is an intelligent invention that be thumbs up. The research found by scientists from Stanford University United States has working similar principle as conventional battery. By using a galvanize battery, the salt water containing sodium chloride will be ionized to positive ions namely sodium and chloride as negative ions. like a flame of light which be a source energy of electric generator. The initial experiment was use a salt water with 200 ml volume, the result is a lamp can be lit for 8 hours. However, after 8 hours, the salt water must be replaced with a new one because the current water can't to generate electricity.
How Can it Light Up ?
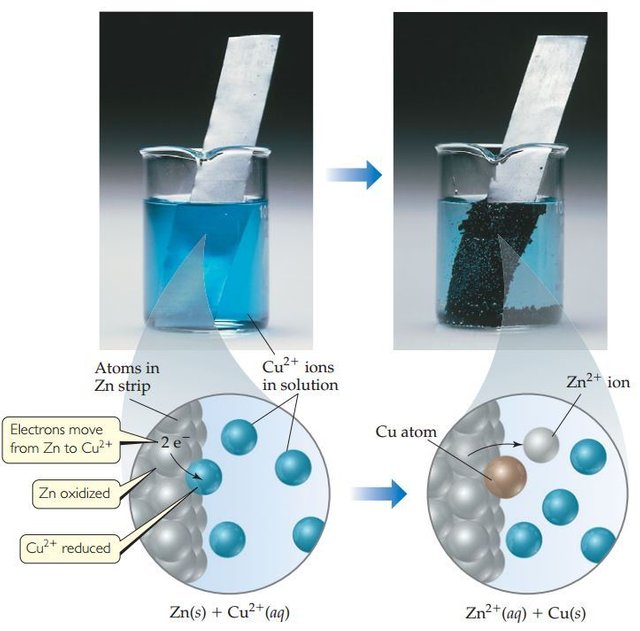
The electrochemical cell comprises of a pair electrodes immersed to a molten or ionic solution then it connected to a metal conductor in outer circuit. The electrochemical cell may be either a voltaic or galvanic cell, and an electrolysis cell. To be understand of voltaic cell, we must first be understood the spontaneous of redox reactions. One example of a spontaneous redox reaction is the reaction of zinc metal with copper (II) sulfate solution. If the shiny gray zinc metal is immersed in a blue copper (II) sulfate solution, gradually, the zinc metal surface will adhere then brownish red copper metal, while blue color of solution will fade. The copper attached zinc metal which derived from solution (a copper ion (II) giving a blue color in water solvent). Meanwhile, the zinc metal forms soluble ionic water, but doesn't give color solution. We can use chemical reaction as bellow :
Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s)
The reaction equation above , the zinc metal is oxidized to form zinc ions (II). This reaction is accompanied by releasing the electrons.
Zn(s) → Zn2+(aq) + e-
The copper (II) ion forms the metal by receiving electrons
Cu2+(aq) + 2e- → Cu(s)
If the reaction is carried out of above manner, the electrons released from direct oxidation reaction are used for the reduction reaction on zinc metal surface. Electrons do not have the opportunity to produce current electric which can to work.
More Explain in Easy Example
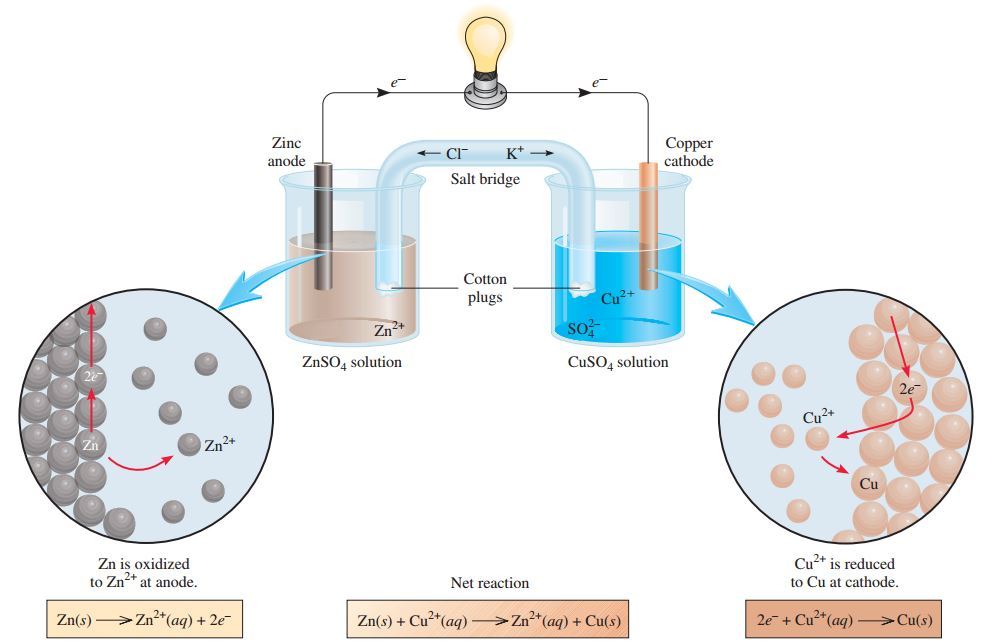
In the Daniell cell, the electrons are designed to flow on outer circuit so that it can to work. The oxidation reaction must be separated from reduction reaction to form the cell. Daniel's cell it means the Volta cell, because Daniell cell is an example of a Volta cell,
Both solutions are connected by salt bridge containing KCl. The electrons flow from Zn electrode (as anode) to Cu electrode (as cathode) through the external wire.
A half of cell is made up of zinc metal dipped in ZnSO4 solution and some other made up of copper dipped to CuSO4 solution. If two electrodes connected to external circuit (e.g cable, wire) will be generate electric current, Which can be evidenced by movement of galvanometer needle attached to outer circuit of the cell or lamp, When Daniell cell used as a source electricity, the Zn changes to Zn2+ which is soluble.
Zn(s) → Zn2+(aq) + 2e- Oxidation
This can be known from decreasing of Zn metal mass. On the other side, The mass of Cu electrode is increasing because precipitation of Cu from Cu2+ in solution.
Cu2+(aq) + 2e- → Cu(s) Reduction
The Zn electrode acts as anode and Cu electrode as the cathode. In electrochemical cell, both voltaic and electrolytic cells, the anode is the electrode where oxidation reaction takes a place and cathode is the site of reduction reaction.
When Daniell cell is used, there is an electron current from zinc electrode (Zn) to copper electrode (Cu) in the outer circuit. In the physics science there is a convention states, the current source of electric flows from a positive to negative pole in outer circuit, or electrons flowing from a negative to positive pole.
Conclusion
Conclusion
The zinc metal acts as a negative and copper metal as a positive pole. At the same time, displacement of some Zn2+ ions from left to right occurs in solution. This is cause left solution is an excess of Zn2+ ions compared to existing SO42- ions. Meanwhile, SOSO42- ions flow from right to the left because the right side of SOSO42- excess with Cu2+ ions.
Source :
Support Scientist By Use #science tag or join @steemSTEM
Follow Me @jamhuery

wah ga kemahalan nanti bikinya mas? garam lagi naik .😂😂
Garam naik di negara kita, mestinya enginer kimia uda bisa turun tangan ni biar garam kita berkualitas
Having chemistry being my worst subject in college I actually remember there redox reaction 😓Lol on that note very cool experiment!
lol, chemistry is a simple science, just memorize every symbol, thanks for reading
Not only does the salt water need to be changed regularly, but the metal electrodes also need changing every so often.
2 less costly metals could make this more cost effective. Maybe aluminum from empty beer and soda cans for one electrode and empty food cans (that are not aluminum) for the other electrode could provide very inexpensive electricity.
There are also many possibilities for the electrolyte solution, like soapy water from showering, or urine, or water with lawn clippings or etc.
While these may be less efficient than the copper-zinc-salt battery, they could be more cost effective.
Exactly, cool and great addition of electric energy source, thanks @point, nice to talk about chemistry
This post has received a 0.39 % upvote from @drotto thanks to: @banjo.