Grottbags Science: Chemistry - Structures and Bonding
The structure and bonding in:
Iodine
is a dark grey/purple crystal solid with a relatively low melting point due to it’s light connections between atoms giving it a fairly weak structure.

Diamond
is known as a Giant Covalent Structure
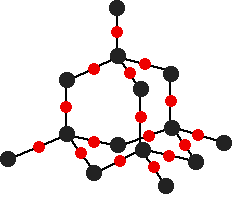
Every carbon atom is covalently connected to 4 more carbon atoms making the bond extremely strong.
This is why diamond has a very high melting point (4000 degrees) because it takes an incredible amount of energy to separate the atoms from there tightly bonded lattice structure.
Diamond doesn’t conduct electricity because all of the electrons are held tightly into place.

Graphite
is also a Giant Covalent Structure made up of carbon atoms, however unlike diamond, these carbon atoms only bond with 3 other carbon atoms leaving the 4th electron in a delocalised state, free to roam across the layers of graphite, making graphite a conductor of electricity.
It is the strong intermolecular forces between each the delocalised electrons, in each layer, that bind the layers of graphite together making it a smooth and soft material whilst also remaining strong like metal.

C60 Fullerene
The Buckminsterfullerene (Bucky Ball)
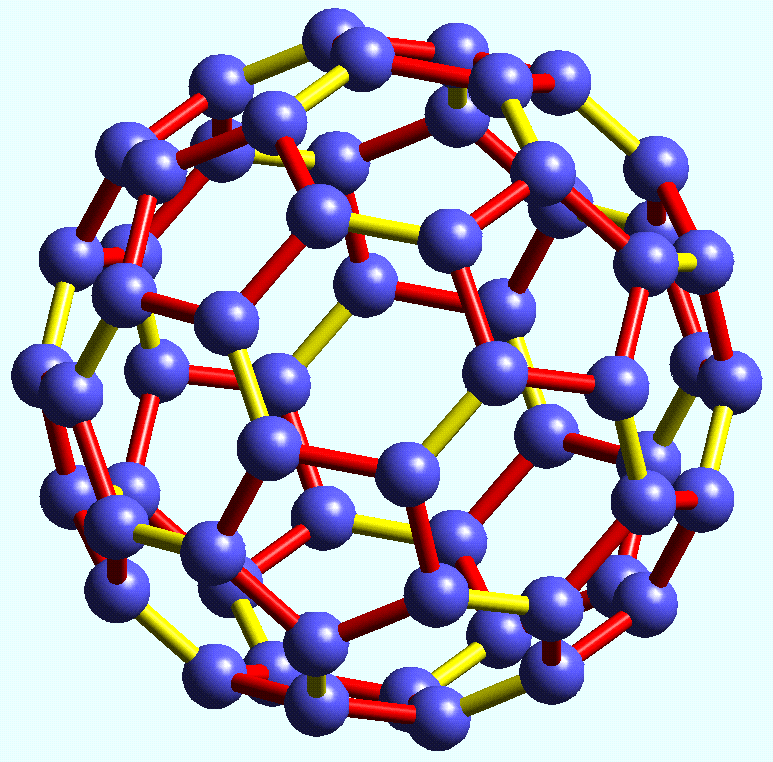
is the arrangement of 60 Carbon atoms in a strong spherical shape. It is similar to that of graphite’s structure with 3 carbon atoms are bonded tightly together with 3 more carbon atoms, however it creates a more tubular (and extremely strong) structure made up of hexagons and pentagons, making this material particularly special for new age technology and advancement.

Carbon Is Amazing!
There has been much work done in recent years on carbon allotropes such as nanotubes and graphene.
Carbon is ‘the most striking material in the universe’.
The discovery of the Carbon atom has revolutionised science and technology to great extents. Carbon is capable of forming many allotropes due to its valency, it has the potential of making a chemically stable, 2 dimensional, one-atom thick membrane in a 3 dimensional world.
Carbon nanotubes are valuable in an extensive range of applications due to their remarkable strength and electrical properties. They have big potential to be used in superconductors, catalysts, drug delivery devices, structural composite materials, flat-panel displays, radar absorbing coatings and biosensors to name a few.
Graphene is an extraordinary material with a broad range of applications in diverse fields such as nanoelectronics, bioenergy, nanobiotechnology, nanosensors, nanocatalysts and many more, due to its high working ability, cost effectiveness and ease of availability.
References/Interesting reads:
Wikipedia.(2016).Allotropes of Carbon.Available: https://en.wikipedia.org/wiki/allotropes_of_carbon.
Santosh K. Tiwari, Vijay Kumar, Andrzej Huczke, R. Oraon, A. De Adhikari, G. C. Nayak.(2016).Magical Allotropes of Carbon : Prospects and Applications.Available: http://www.researchgate.net/publication/285089938_magical_allotropes_of_carbon_prospects_and_applications.
Save a tag for 'steemstem' !! An important tag for posts covering topics in the STEM fields with an active userbase
Congratulations @grottbags! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOP