Why some liquids float on water..
We will first talk about the density of water..
The density of water is the weight of the water per its unit volume, which depends on the temperature of the water.
Liquid type Density
Lamp oil ** 0.80 g/cm^3**
Rubbing alcohol 0.87 g/cm^3
Vegetable oil ** 0.91 g/cm^3**
Water 1.0 g/cm^3
Water has the highest density why others have lower density
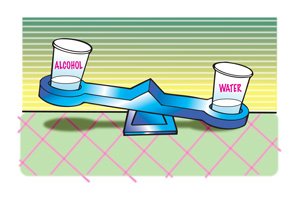
Which liquid is more dense?
We should agree that the water is more dense than the alcohol.
How do we know?
Since the water has more mass than an equal volume of alcohol, water must be more dense.
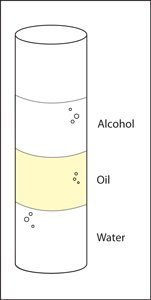
In the above picture of of a graduated cylinder we have 15ml of alcohol, 15ml of oil and 15ml of water ..
This expected result is...
Alcohol floats on oil and water sinks in oil. Water, alcohol, and oil layer willl because of their densities, but also because the oil layer does not dissolve in either liquid. The oil keeps the water and alcohol separated so that they do not dissolve in one another.
Why does the alcohol float on the oil?
Why does the water sink in the oil?
Water sinks because it is more dense than oil. ,just like solids, liquids are made from atoms and molecules, which have a certain mass and size. Depending on the mass of the molecules that make up a liquid and how closely they pack together, liquids have their own densities.
In the activity, we will compare the mass of equal volumes of each liquid. Which liquid should have the most mass? The least mass? In between?
water will weigh the most, the alcohol will weigh the least, and the vegetable oil will weigh somewhere in between.
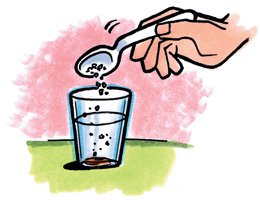
Let's take a look at a slice of carrot and water... Will the carrot float or Not??
Carrot will sink in water as we can see in the image below..
But by adding salt to the water the carrot will therefore float on the water..
Dissolving salt in water increases both the mass and volume of the water, but it increases the mass more. Because D = m/v, increasing the mass more than the volume results in an increase in density. D=density , M =Mass and V=Volume
therefore we will conclude that the carrot is less dense than saltwater
Lastly If equal volumes of water and saltwater were placed on a balance, the saltwater would be heavier.... You can also add your own point in the comment box below . Thanks.

Nice article. Keep it up
Thanks bro
Nice write up! I now know more about density of water and alcohol
Well written article very informative
Well written. Steem on!