The ideal gas law
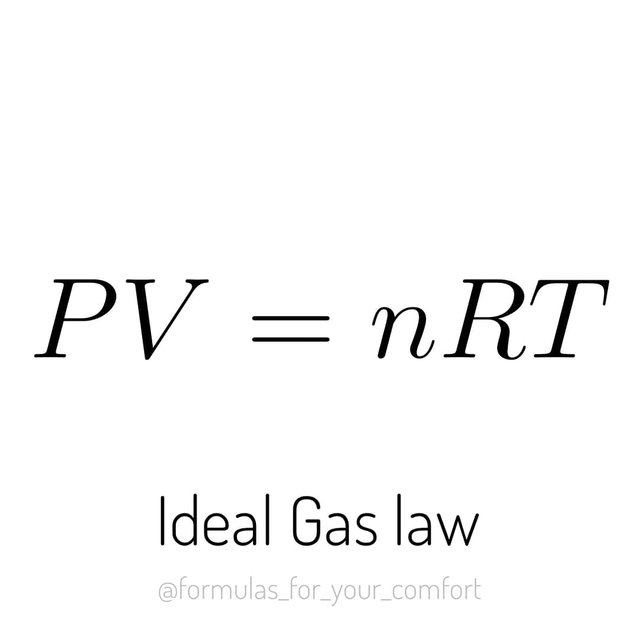
The ideal gas law is an important relationship that describes the state of an ideal gas.
An ideal gas is a theoretical gas where we neglect both molecular size and inter molecular attractions, this is of course not exactly how things happen at a microscopic level but it makes a realistic approximation for many different gases which allows for easier calculations.
P is the pressure of the gas, V its volume and T its temperature. Three important variables that define the state of a given gas.
R is a constant called the ideal gas constant, 8.314 J/(K.mol) and n is the amount of substance of gas in moles.
This approximation works best for monoatomic gases at low pressures where the fact that we neglect the molecular size is the most realistic and since different molecules have a large space to roam, the interactions between them can be neglected.
Do you know the more general formula, for real gases ?
More @formulas or on formulas_for_your_comfort
This post has been voted on by the SteemSTEM curation team and voting trail in collaboration with @curie.
If you appreciate the work we are doing then consider voting both projects for witness by selecting stem.witness and curie!
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
✅ Enjoy the vote! For more amazing content, please follow @themadcurator for a chance to receive more free votes!
Congratulations @formulas! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
Click here to view your Board
If you no longer want to receive notifications, reply to this comment with the word
STOP