Chemistry Express: The Periodic Table

Everything you see around you, all the buildings, cars, animals, your own body and all the stars in the night sky, are all made by the 118 elements of the periodic table. How is that possible? These elements combine in different ways under different circumstances, which creates compounds with unique properties!
The periodic table of the elements is one of the most important tools of chemistry. It helps us realize that the chemical elements are not just a bunch of random substances; they follow trends and make up families with similar characteristics. Each element is defined by the number of protons in its atoms. The periodic table starts with Hydrogen, with one proton, followed by Helium, with two protons, and so on.
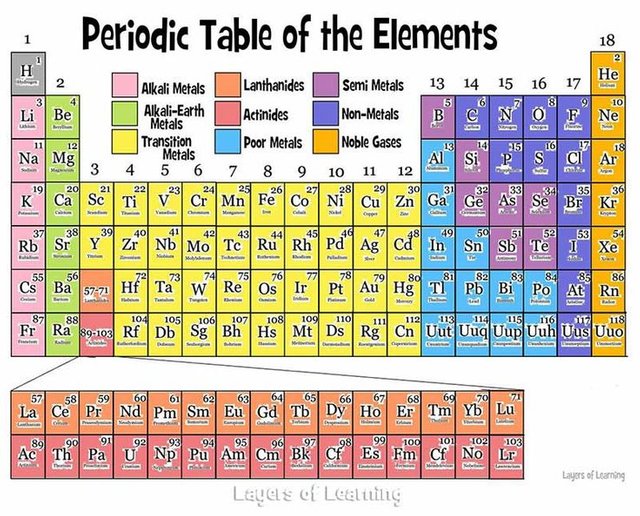
image credit
As we mentioned in a previous post, every atom really wants to have eight electrons in its outermost shell, called valence electrons, in order to be more stable. Elements that belong to the same vertical column, or group, are elements with the same number of valence electrons. Elements that belong to the same horizontal row, called a period, are elements which their atoms use up the same number of electron shells.
As we move from left to right on a period, we observe a gradual transformation of properties. Each period starts from a very reactive metal and ends in a very reactive non-metal, followed by an inert gas. Between metals and non-metals we can see the metalloids, which display variances of both properties.
We can also observe similarities between the elements that belong to the same group. Alkali metals, for example, are on the first group and have one valence electron. Having only one v. electron means that it’s easier to give it up in order to have a full electron shell as the last shell. That is why the alkali metals are very reactive, since they are constantly looking for a chance to give up their only valence electron! Alkali metals react with pretty much everything, even with water, and this is why they can rarely be found in their pure state.

image credit
It’s important to note here that hydrogen is an exception, since it has only one electron and the first shell only needs two electrons to be filled. That means that hydrogen doesn’t necessarily need to give up its electron; it can as easily borrow one from another element and fill its small shell. This, for example, is how a methane molecule is created. Methane is made of one carbon and four hydrogens. As we can see on the periodic table, carbon has four valence electrons; the four hydrogens have one each. They share their v. electrons so that it’s as if the carbon had eight and as if each of the hydrogens had two!
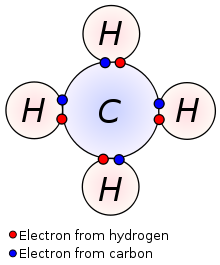
image credit
Alkaline earth metals are almost as reactive. They can be found on the second group and have two v. electrons. These are also rarely found in a pure state. In the next nine groups (groups 3-12) are the transition metals. In reality, a metal property is defined by how likely is the element to give away an electron. It’s just easier to give them when you only have a few of them.
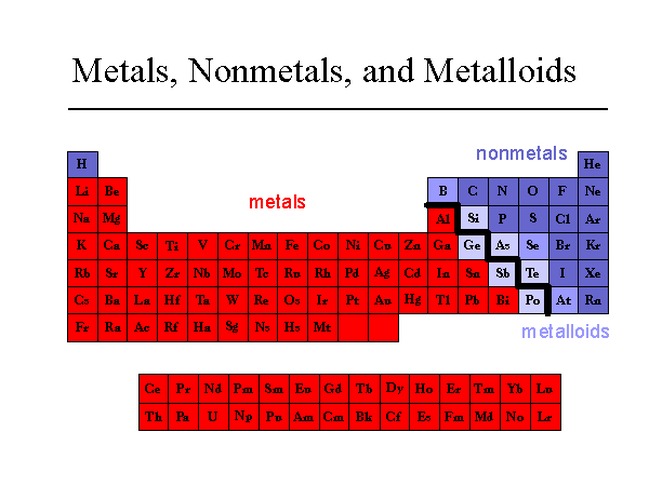
image credit
The transition metals in principle have two valence electrons. However, due to the complicity of shape of their electron orbitals, the highest energy electrons of transition metals are not in the outermost shell but in the previous one, as we mentioned in the previous post. That means that these metals have a lot of “backup” electrons which can also participate as v. electrons.
For this reason metals have a lot of v. electrons. For example gallium, even though it’s a “poor” metal, it has three electrons in its outermost fourth shell, but it also has ten high energy electrons in its third shell for backup. Therefore, a gallium atom ends up sharing those electrons with more gallium atoms. This is what causes metals to conduct electricity really well, as the electricity is really nothing more than electrons moving. That’s also what’s causing them to be malleable and ductile.
Lanthanides and actinides belong to the sixth and seventh period and in reality they should be where lanthanum and actinium are on the periodic table. We only place them outside of the main body of the periodic table for efficiency purposes, to avoid crowding.
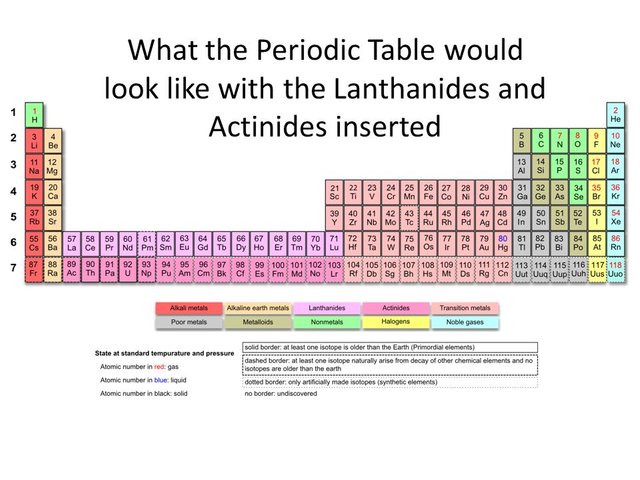
image credit
Group 13 elements have three v. electrons, which means it’s still easier for them to give them up but there are cases where they choose to take instead of giving to complete their octet. Group 14 has four v. electrons, and since it's only half from being a full octet, giving or taking depends on the individual element. Carbon finds it easier to gain four electrons, while lead finds it easier to give, since lead has more shells and its v. electrons are further away from the nucleus, rendering them easier to remove.
The elements of groups 15 and 16, the non-metals, have five and six v. electrons respectively. That causes them to want to gain electrons instead of giving in most cases. The elements of group 17 have seven v. electrons. These are called the halogens, and since they are only one electron away from completing an octet, they especially tend to react with alkali, which want to give their only v. electron! For example, chlorine, a halogen with seven v. electrons, likes to react with potassium, an alkali with one v. electron. Potassium will pass it over to chlorine, and will remain with eight v. electrons, it’s third shell now being the last one.
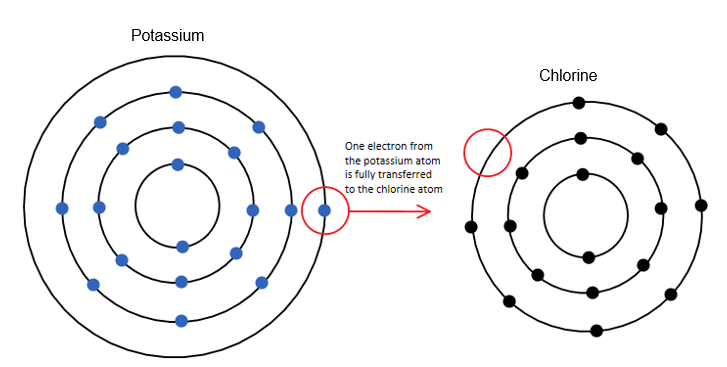
image credit
Finally, we have the noble gases on group 18. These elements are not interested in reacting, since they have their shells full. That’s why they are usually inert and stable. Now we know why we get helium and not hydrogen balloons!
From the above we can see that the periodic table is very useful to help us study the properties of elements, predict their behavior and common characteristics, and find out with which other elements they are more likely to react and form bonds.
Here is an interactive periodic table if you want to explore the elements. You can click on an element and find out many interesting facts about it!
And here is a fun song to help you remember all the elements, if you want to memorize them!

Hey, great post! We have featured your article in our weekly newsletter! We apologize that the payout has passed for this post, but we still wished to feature your content regardless. Keep up the good work, and let us know if you have any concerns.
Join us on the science-trail Discord
I just saw this message. Thanks a lot for featuring my article! I will join you on Discord :)
Thank you so much for this informative post! Upvoted :)
Keep it up!
Thank you! :)
Looks seems good.nice share about chemistry..vote my article too about time machine,and follow @rezafrizayya,i will foll back
Thank you!
Hi~nice to see you:)
hello :)
Yay, more chemistry geekery, thanks! ^_^
hehe yep! thank you :)
Thank you! I followed you :)