Learning Physics : Effect of pressure and impurities on boiling point
Hello all steeamians lover. On this occasion lets learn together about physics lesson. Hope you easy to understand on this topics above.
a. Effect of pressure on boiling point
Have you ever gone camping in a mountainous area? Have you ver tried to boil water there? If you did, you would find that the time needed to boil water in the mountainous area is faster than t needed to boil water in the seashore. Why does it happen???
The boiling point of liquid is influenced by the air pressure above the liquid's surface. When the air pressure decreases, the boiling point also decreases. The air pressure in the plateau (mountainous area) is lower than the lowland (seashore), so the boiling point in the plateau is lower than that in the lowland which cause the water boil faster.
The boiling point of water at normal pressure mHg is 100°C. If the pressure decreases then of 76 water will boil at a temperature lower than 100°C. The boiling point of water decreases 1°C every 300 m of height increase above sea level.
To prove the statement above, perform the following experiment!
Prepare two containers, and then fill them with the same amount of water (Figure 1). The first container is equipped with a thermometer and an air pump (Figure 1.a), while the second container is only equipped with a thermometer.

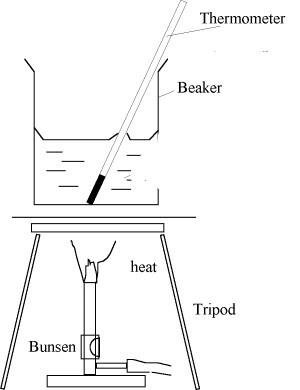
When the air is pumped out, the air preasure decreased so that boiling point in (a) lower than the boiling point in (b)
The air above the water's surface at the first container is pumped out, which causes the air pressure decreases. Then both containers are heated at the same time with the same flame. We will find out that the boiling temperature of the first container is lower than that of the second one. It indicates that the boiling point of liquid is affected by pressure.

One of the uses of the pressure effect on boiling point in our everyday life is a pressure cooker (Figure 2). The pressure cookers lid is able to hold down the water steam from getting out the cooker, which make the pressure inside the cooker increase. As a consequence, the water boils at a temperature above 100°C. A high boiling point makes foods, i.e meat, cooked faster.
b. Effect of impurities on Boiling Point
What is the effect of impurities on the boiling point of water? Let us find out the answer through the following experiment.

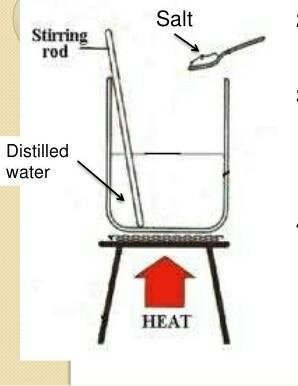
When we add salt in container 2, the boiling point of container 2 will bigger than container 1
On Figure 3 we can see two heat resistant containers contain pure water with the same volume. We add sugar or salt on the second container. Next, those containers are heated at the same time with the same flames. Wait until the water boils, then measure the temperature of each container! Do they have the same temperature?
The water containing impurities (sugar or salt) has greater boiling point than the pure water does. Thereby, impurities are able to increase the boiling point of a substance.
Sources :
What effect does pressure have on boiling point?
