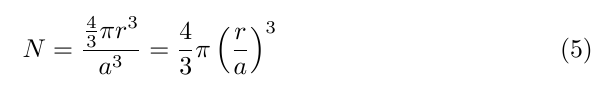Semiconductor Physics: Part 1 - Nanostructures
Preface
In this series of posts, inspired by a lecture course given by Dr. Phil Buckle, we will develop an understanding of the concepts that underpin the exciting field of low dimensional semiconductor devices. While some of this treatise is mathematical, it is my hope that this work will bring value to readers of all abilities.
Quantum Confinement
We begin by considering the notion of quantum confinement.
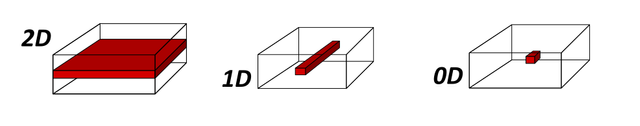
FIG. 1: Different degrees of quantum confinement
2D confinment, 1D confinement and 0D confinement characterise quantum wells, quantum wires, and quantum dots, respectively. This can be a confusing concept, so it is important to remember that the dimensionality of this description refers to the dimension the system is confined to. This convention is used extensively throughout the literature, so it is important that this is understood.
Surface to Volume Ratio
- This is extremal for nanostructures. At smaller length scales, one would be considering just a system of atoms. This gives rise to the term extreme functional material, to which nanostructures are often referred to.
- The pros of nanostructure materials are that they make good catalysts, due to the high surface to volume ratio, as well as being useful for application in plasmonic and coatings.
- The cons are that properties of the material may be dominated by undesirable surface effects, for example, carrier loss in optical systems.
Quantum Well Confinement
For a quantum well of length l and width a, where l>>a, the surface to volume ratio is given by
Quantum Wire Confinement
For a quantum wire of length l and radius r, where l>>>>r, the surface to volume ratio is given by
Quantum Dot Confinement
If we model a quantum dot as a cube of dimension a , then the surface to volume ratio is given by
while if we model a quantum dot as a sphere of radius r, the surface to volume ratio is given by
In each case, the inverse power law with nanoscale dimension is critically controlling the surface to volume ratio.
Number of Atoms
So how many atoms do we expect?
Let's consider a 10 nm, gold nanoparticle. Now, this has an FCC lattice structure (4 atoms/unit cell), with a unit cell size of ~ 0.408 nm. We determine the number of unit cells, N by dividing taking the ratio of the sphere volume to the unit cell volume
which leads us to a value of ~247,000 atoms.
Now, as the size of the nanoparticle decreases, the number of consituent atoms decreases dramatically: a 5 nm gold nanoparticle, for example, constitutes ~31,000 atoms.
The majority of the atoms in a given nanostructure reside at the surface.
Length Scales
Planck was the first to put forward the relation between the momentum of a particle and its corresponding wavelength, understood through wave-particle duality
The de Broglie wavelength is what we refer to when we talk about a quantum wavefunction. A free electron has a de Broglie wavelength of ~0.24 nm.
As a fun example, if we consider a person of mass 70 kg, walking at 1 m/s directly into a wall - their de Broglie wavelength is ~9.5 x10^-36 m. This means they will walk right into the wall (ouch) rather than tunnelling through it!
The de Broglie wavelength of a semiconductor electron, however is ~73 nm, and for a semiconductor hole is ~18 nm. This is good news.
In the next post, we will discuss this further.