Ivermectin as a COVID19 Prophylaxis
Although I usually don’t make separate posts for edits to my answers, I was not aware of this body of evidence for Ivermectin’s efficacy against COVID19 at the time I posted this answer last year. Kory and colleagues published a systematic review of the evidence that Ivermectin is an efficacious prophylaxis for COVID19, in the American Journal of Therapeutics, including 18 RCTs and several other non-randomized and unblinded prospective and retrospective control trials in both humans and animals. I excluded a handful of case series studies listed in their review, which didn’t have a control group or competing intervention for comparison, as well as an observational control trial conducted in Brazil that did not provide primary data, and the cell culture study that every other study cited but which used a much larger dose than what would be used clinically.
Human Studies Included:‘Elgazzar and colleagues at Benha University in Egypt randomized 200 health care and household contacts of patients with COVID-19 where the intervention group consisted of 100 patients given a high dose of 0.4 mg/kg on day 1 and a second dose on day 7 in addition to wearing personal protective equipment, whereas the control group of 100 contacts wore personal protective equipment alone. They reported a large and statistically significant reduction in contacts testing positive by Reverse Transcriptase Polymerase Chain Reaction (PCR) when treated with ivermectin versus controls, 2% versus 10%, P < 0.05.’
Another RCT conducted at another Egyptian University (n = 340):Found a statistically significant decrease in COVID19 symptoms among patients treated with 0.25 mg/kg Ivermectin administered twice on the day of their positive test and 3 days afterwards compared to the control group: ‘After a two-week follow-up, a large and statistically significant decrease in COVID-19 symptoms among household members treated with ivermectin was found, 7.4% versus 58.4%, P < 0.001.’
A prospective observational trial conducted among healthcare workers in Bangladesh (n = 118):Found that those who took 12 mg Ivermectin orally for 4 months were far less likely to test positive for COVID19 than healthcare workers in the control group. ‘73.3% (44 out of 60) subjects in the control group were positive for COVID-19, whereas only 6.9% (4 out of 58) of the experimental group were diagnosed with COVID-19 (p-value < 0.05).’
Another prospective observational trial conducted by Carvallo and colleagues (n = 229):Found that healthcare workers who took 0.2 mg ivermectin five times daily for 28 days were far less likely to test positive for SARS-COV-2 compared to healthcare workers who volunteered for the control group: ‘After 28 days, none of those receiving ivermectin in the prophylaxis group had tested positive for SARS-COV-2 versus 11.2% of patients in the control arm (P < 0.001).’
A follow-up prospective observational control trial (n = 1,195)conducted over a 3 month period also found no positive tests among healthcare workers administered 12 mg Ivermectin weekly, while 58% of the control group tested positive for COVID19.
A separate replication of their protocol in an RCT conducted by the Health Ministry of Tucuman in Argentina (n = 234)found that only 3.4% of healthcare workers who took 12 mg of Ivermectin weekly contracted SARS-COV-2 compared to 21.4% of healthcare workers in the control group.
A retrospective case control study among healthcare workers within the India Medical Institutes of Sciences (n = 372):Found that workers who took a regimen of 300 micrograms/kg Ivermectin one day one and four and the same dose once a month, had a 73% reduction in SARS-COV-2 infections the following month compared to workers who took other treatments including HCQ, Vitamin C and a single dose of 300 micrograms/kg dose of Ivermectin.
An analysis of a prophylactic Chemotherapy database administered by WHO, case counts obtained by worldometers, and a public data aggregation site used by John Hopkins University:Found lower COVID19 case counts in countries with mass drug administration programs for Ivermectin than countries that administered some prophylactic chemotherapy and countries that administered none.
Another RCT conducted in Bangladesh at Dhaka Medical College (n = 363):Found that participants administered 2x 6 mg doses of Ivermectin and 100 mg Doxycycline were less likely to require more than 12 days for clinical improvement (23%) compared to the placebo controls (37.2%) were less likely to deteriorate to more severe COVID19 disease (8.7%) compared to the placebo controls (17.8%), were less likely to remain SARS-COV-2 positive 14 days after their initial positive test (7.7%) compared to placebo controls (20%). The Ivermectin plus Doxycycline also had zero deaths compared to the three deaths in the placebo control group.
Another RCT conducted in Eastern India (n = 115):Found that patients treated with 12 mg Ivermectin on the first two days of enrollment in the study were more likely to be discharged (100%) compared to the placebo controls (93%) over the 2 month study period; 6.9% of participants in the control arm died in the hospital. This difference was found to be statistically significant at the 0.05 alpha level.
Another RCT conducted in Lagos, Nigeria (n = 62 ):Found a statistically significant difference in viral clearance between the low dose (6 mg IV 2x Week) and high dose (12 mg IV 2x Week) treatment groups at the 0.01 alpha level. Participants in the 2 Ivermectin arms of the study were 3.45x more likely to test COVID19 negative by day 5 compared to the control arm who received Lopinavir/Ritonavir which was statistically significant at the 0.05 alpha level.
Another RCT conducted in Baghdad (n = 140):Found that patients with mild to moderate disease treated with 200 micrograms/kg daily for 2-3 days in combination with 100 mg doxycycline 2x daily for 5-10 days had half the mean time to recovery (6.3 days) compared to patients treated with the standard of care of 40 mg methylprednisolone or 6 mg dexamethasone along with 1,000 mg vitamin C and 75–125 mg zinc 2x daily as well as 250 mg azithromycin and 5000 IU Vitamin D3 daily (13.7 days). This difference was statistically significant at the 0.0001 alpha level. Among patients treated with the Ivermectin regimen only 4.3% progressed to more severe disease compared to 10% of patients in the standard of care group and reduced mortality rate of patients in more advanced stages of disease from 22.7% to 0%.
A smaller RCT conducted in Spain (n = 24):Found that patients treated with a single 400 microgram/kg dose of Ivermectin had a significantly lower median viral load by 3x at day 4 and 18x by day 7 compared to the placebo control group. Patients in the ivermectin group also reported 50% less anosmia/hyposmia and 30% less cough symptoms compared to placebo controls.
Another RCT conducted in Bangladesh (n = 62):Found that patients administered a single 200 micrograms/kg dose of Ivermectin had a 1.5 days shorter mean recovery time (10 days) compared to patients in the control arm (11.5 days) although this difference was not statistically significant compared to the standard of care.
Another RCT conducted in Bangladesh (n = 116)also found that participants administered a similar single dose of Ivermectin had a 1 day shorter mean time to symptomatic recovery (5.9 days) compared to the placebo group (7 days) although this also was not statistically significant at any alpha level.
Another RCT conducted among hospitalized patients in Dhaka, Bangladesh (n = 72):Found that patients treated with a 5 day course of 12 mg oral Ivermectin had a shorter mean duration to viral clearance (9.7 days) compared to patients who received the same 5 day Ivermectin regimen in combination with 2x daily doxycycline (11.5 days), and the placebo arm (12.7 days). Virological clearance after the 5 day Ivermectin regimen was significantly earlier compared to the placebo arm on days 7 and 14 of the study at the 0.05 alpha level.
An Open label retrospective cohort study conducted here in the U.S. in 4 Broward (County) Health-associated hospitals (n = 280):Found that patients treated with at least one dose of 200 micrograms/kg Ivermectin on their first day and a second dose on day 7 (n = 13), at the discretion of the treating physician, had an overall (statistically significant) mortality rate (15%) compared to patients treated with other medicines mostly corticosteroids (25.2%). Among patients with severe pulmonary involvement, treatment with at least one dose of Ivermectin, but no more than two doses, cut the mortality rate in half from 80.7% to 38.8%. This difference was statistically significant at the 0.001 alpha level. In a matched cohort, Ivermectin treatment was associated with an absolute risk reduction of 11.2% and 8.9 patients needed to treat to prevent one death.
An RCT conducted in Iranfound that patients receiving numerous different Ivermectin regimens had a mean COVID19 mortality of 3.3% while patients in the placebo arm receiving the standard of care had a 5.7x higher mortality rate of 18.8%.
Another RCT conducted with hospitalized patients in India (n = 100):Found that patients given a combination of Ivermectin and Doxycycline (no dosage listed) had a shorter hospital stay (3.7 days) compared to patients given a Vitamin B6 gel as a placebo (4.7 days) and a shorter time to complete resolution of symptoms (6.7 days) compared to the placebo control arm (7.9 days). These differences were statistically significant at the 0.05 and 0.01 alpha levels respectively.
A retrospective cohort study conducted at Mymensingh Medical College Hospital in Bangladesh (n = 248):Found that patients treated with 12 mg Ivermectin within 24 hours of hospital admission had a significantly lower mortality rate (0.9%) compared to patients treated with the standard of care (6.8%). 46% of patients in the control arm required oxygen compared to only about 10% of patients administered Ivermectin. Fewer patients treated with Ivermectin developed respiratory distress (2.6%) or required intensive care (0.9%) compared to patients who received the standard of care (15.8% and 8.3%). Patients treated with Ivermectin also had a shorter median duration of hospital stay (9 days) compared to patients who received the standard of care (15 days) and tested SARS-COV-2 negative much faster (median 4 days) than patients treated with the standard of care (median 15 days). These differences were statistically significant at the 0.001 alpha level.
A pilot clinical trial conducted at Al-Shifa’a Hospital Center in Iraq (n = 87): ClinicalTrials.govFound that patients treated with 200 micrograms/kg Ivermectin at admission to hospital along with a regimen of HCQ and AZT for their first 5 days had a shorter mean hospital stay (7.6 days) compared to patients in a synthetic control arm that received the HCQ and AZT alone (13.2 days) and were PCR positive for fewer days (median 7 days) compared to the synthetic control arm (median 12 days).
A double blind placebo controlled RCT, conducted in Iran between July 2020 and June 2021, with unvaccinated adult patients with moderate to severe COVID19 disease (n = 110) found that patients administered 14 mg Ivermectin twice daily for the first 3 days of their illness were significantly less likely to be admitted to the ICU and intubated and had a shorter hospital stay and symptom duration compared to patients treated only with the standard of care plus a true placebo. The standard of care included 200 mg of Remdesivir on day 1 and 100 mg daily over 5 days along with a daily corticosteroid dose. 32.7% of patients in the placebo arm had to be admitted to the ICU compared to 5.5% of patients treated with Ivermectin. 20% of patients in the placebo arm were intubated compared to only 3.6% of patients treated with Ivermectin. Patients in the placebo arm were hospitalized for a median of 6 days compared to a median of 4 days in the Ivermectin arm who also had a shorter median duration of symptoms of only 3 days compared to 4 in the placebo arm.
This is not the first RCT to find that Ivermectin has virologic effect against COVID19. As I mentioned in Ivermectin as a COVID19 Prophylaxis another 13 RCTs and several other observational studies with different levels of control have also found that Ivermectin effectively treats moderate to severe COVID19 disease while several large clinical trials, mainly conducted in the U.S., have found it to be ineffective. However, as Dr. Goodkin, a fellow of the American College of Cardiology, recently noted these trials started treatment or randomization an average of 5 days after symptom onset while the drug needs to be administered within the first 3 days for maximum efficacy. They also administered the regimen to patients on an empty stomach minimizing absorption, limited titration for obese patients at the highest risk, and administered doses that were only a fraction of the Frontline COVID19 Critical Care Alliance recommended cumulative dosage, changed clinical endpoints mid trial in one, and administered Ivermectin as a standalone treatment instead of as a regimen with other drugs and Zinc. The PRINCIPLE trial I covered previously didn’t randomize participants into Ivermectin and placebo arms until day 14 but still found statistically significant differences in clinical endpoints and dismissed them as not clinically meaningful. The COVID-OUT trial I covered last week administered it as a standalone for a much shorter duration than the metformin they found reduced viral load and rebound.
A retrospective cohort study of excess deaths in Peru (n = 33 million):
Found a 13x increase in excess deaths after restrictions on the distribution of IVM were put in place and a 74% reduction in excess deaths after the 30 day peak in the states with maximal distribution compared to 53% reduction in medium distribution states and the 25% reduction in Lima. This is circumstantial evidence for lower mortality with Ivermectin treatment following covid infection.
In Brazil the cities which began a massive Ivermectin distribution campaign in the spring and summer of 2020 particularly the cities of Itajai, Macapa, and Natal had large decreases in case counts afterwards:
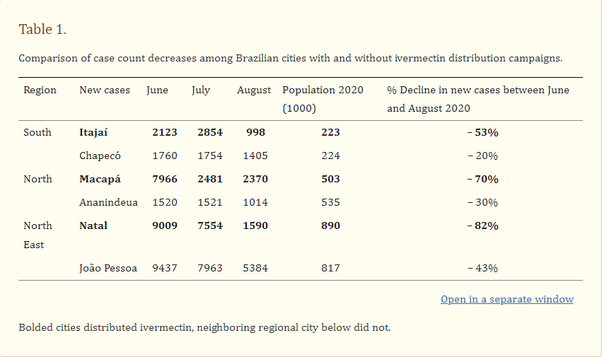
These campaigns were also associated with a sharp reduction in mortality rate:
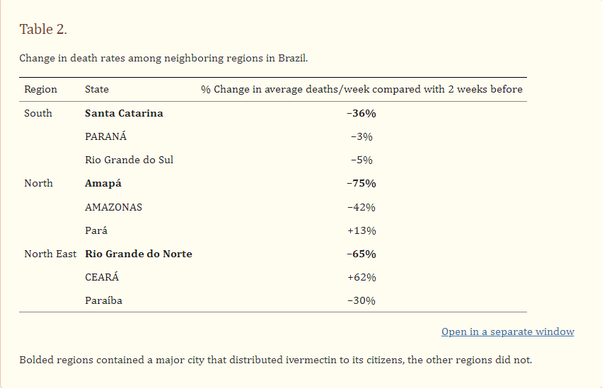
Rodent Studies:
A Syrian Hamster model study:Found that hamsters infected with SARS-COV-2 after a single 1mg/kg dose of Ivermectin had 10x lower antibody titers in their lungs, indicating a reduction in pulmonary injury, and higher Plasma naB titers 10 days after initial infection as well as higher zinc concentrations in lung suspensions by day 3 of the infection. Ivermectin administration also suppressed the expression of proinflammatory cytokines.
A Mouse model in vivo study:Found that mice administered a single dose of Ivermectin after infection with mouse hepatitis virus had lower viral loads in their livers (via Higher Cycle Threshold Values in qPCR) compared to mice infected with the same virus without treatment and had reduced TNFα cytokine levels five days after infection. These findings are pertinent to COVID19 because mouse hepatitis virus enters the cells’ nucleus through the same mechanism as SARS-COV-2.