Do you know semiconductors? your answer is no... "I will explain to you very easily" Part # 4
Hello friends, today I want to continue teaching all semiconductor compounds. We have already learned a little about the basic concepts, ie the theory involved in the physical behavior of these materials. But you ask yourself: how are these compounds made? Of the periodic table of elements? How? When? All these questions will be clarified in the course of the days as my person publishes more material on this interesting topic.
We all know that they are semiconductors and so they are used, but few know how they are made, or maybe if they have idea, but they have no notion of how being a simple pure element found in nature happens to be an electronic device, obviously that to develop an electronic device you need to learn a lot of engineering and electronics. But we physicists study everything from the beginning, and then provide them with the right tools for the engineer to apply.
There is a lot of physics involved in semiconductor compounds, of course !! And you need to study certain physical properties in order to understand their behavior, and to see if they have applications in the future. We know metals that are excellent conductors of electric current as they are: gold and silver but as they are very expensive and difficult to find in nature, we have been forced to use copper, which is much smaller conductor than the first two, but that their studies for years reveal that it is suitable for the construction of cables, coils, among others.
Now there are different families and groups of semiconductors of the periodic table that can be synthesized, that is to say to grow these elements that result in a compound, where after obtaining the ingot or sample of the same, will be made physical studies to observe its properties.
There are binary, ternary and quaternary compounds, although few know that the semiconductor physicists were able to synthesize two different elements and turn them into one, and then the thirst of the scientist and curiosity made them synthesize 3 and 4 elements in a single semiconductor compound and here it all begins:
There are simple semiconductors with silicon (si) and germanium (ge); binary compounds as in the family III-V and III-VI: AlP, AlAs, Alsb, GaN, Zn, ZnS, ZnSe, ZnTe, among others; Ternarios such as CuInTe, CuInSe, CuGaTe, AgGaTe, among others; quaternary: CuInFeTe, CuFeAlTe among others. They are the most known and studied in the literature.
In order to be able to join compounds, specialized techniques are needed for their growth synthesis. In this post I will tell you about the technique used to synthesize copper-indium-tellurium (CuInTe) and it is the following:
Technique of telurization of Cu and In in the molten state.
To grow these elements used the technique called HORIZONTAL GRADIENT FREEZE which consists of the selenization of Copper and Indian in stoichiometric ratio in liquid phase and its subsequent programmed cooling. Depending on the evaporation temperature of selenium, ingots have been obtained, most of these monocrystals with stoichiometry close to 1: 1: 2. You will wonder what stoichiometry is right ?. In a simple way it could be said that it is the proportion or percentage of the elements used to grow the sample of the semiconductor compound, that is to say if you have a 1: 1: 2 stoichiometry this means that you have two elements with 25% of proportion and another with a 50% share; together the three elements make a total of 100% stoichiometric ratio.
The synthesis of this compound was carried out in a three-zone furnace which can be controlled separately as shown in (Figure 1).
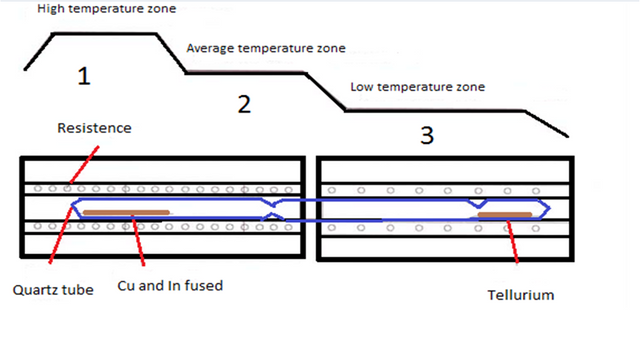
Figure 1: Profile of the synthesis oven.
And the steps for the synthesis were the following:
To perform the synthesis, a high vacuum (10exp-5 torn) test tube containing pure elements (5N-6N) tellurium on one end and copper and indium on the other is placed in this furnace. Thus tellurium is located in zone 1 and copper and Indian in zone 3 of the oven.
First zone 3 is heated up to 1100 ° C so that copper and indium are mixed in the liquid state.
Zone 1 is heated to a certain temperature for the evaporation of tellurium.
The tellurium vapor is reacted in zone 3 with copper and indium "molten" to form CuInTe2 in liquid phase.
Once the reaction is complete, the gradient is established for CuInTe2 growth.
In this way several ingots with temperature between 480ºC and 690ºC were obtained. proceeding to the structural, optical and electrical characterization of these ingots.
Later I will show you what these characterizations are and the different techniques used, but before it is very important to have basic knowledge about the theory.
In my next post I will speak a bit of crystalline structural, next of the structural characterization of semiconductors by means of the x-ray diffraction.
The publication was made under the consent of my tutor Dr Giovanni Marin
Sources:
Rogacheva, I. (1997). Nonstoichiometric in the I-III-VI2 compounds.11th International conference and ternary and multinarycompound, ICTMC-1.Edited R,D Tomlinson. University of Salford
Marin, G. Wasin M,S. Sanchez, G. Perez. Mora, A. (1998). Caracterización estructural y de composición del CuInTe2 obtenido por la técnica de evaporación del Te.CIENCIA 6(2), pag 129-137
I hope you liked my post until you say goodbye Carlos Pagnini